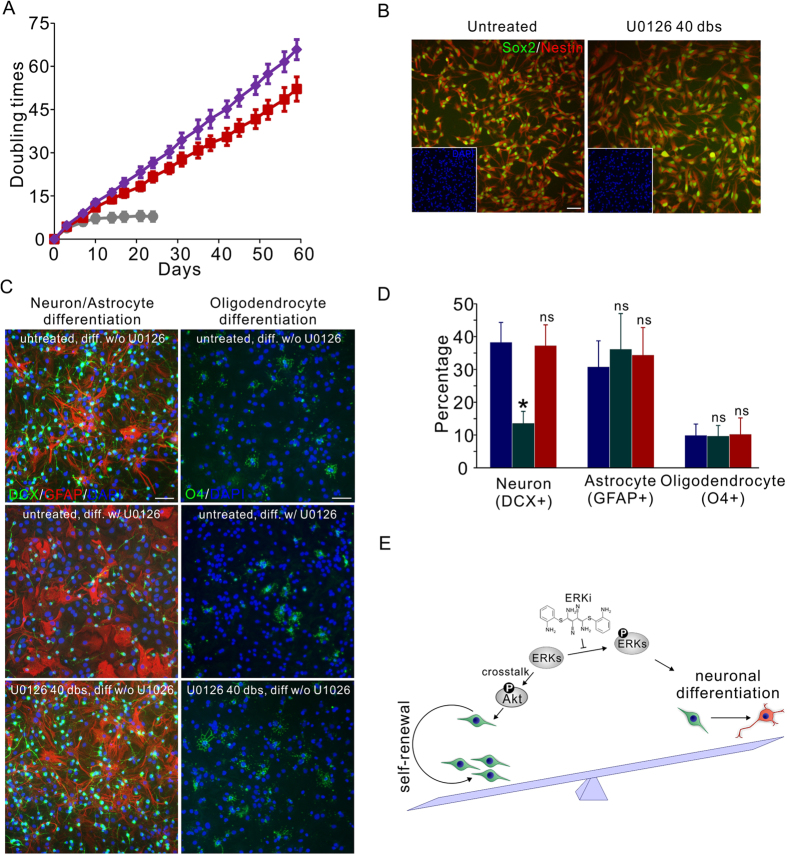
Figure 4. ERKi regulates the self-renewal and differentiation balance of NP cells in vitro.
(A) Continuous ERKi treatment (3 μM U0126) led to the prolonged expansion of monolayer cultured Rat CX cells at a stable doubling rate of about 27 hours (red). Combination with GSK3i (1 μM BIO) further reduced the cell cycle time to 22 hours (purple). Untreated cells suffered significant cell cycle arrest along with expansion (grey). Mean ± s.d., n = 3. (B) Rat CX Cells expanded with ERKi for 40 doublings (dbs) retained homogeneous expression of NP cell markers Sox2 and Nestin. (C) After ERKi removal, the ERKi treated cells can be differentiated into neurons (DCX+), astrocytes (GFAP+) and oligodendrocytes (O4+) (bottom, U0126 40 dbs, diff w/o U0126), indistinguishably from the untreated cells (top, untreated, diff w/o U0126). The presence of ERKi during differentiation significantly inhibited the neuronal differentiation but not glial differentiation (middle, untreated, diff w/ U0126). Scale bars, 50 μm. (D) Quantification of the percentages of neurons, astrocytes and oligodendrocytes generated by untreated Rat CX cells differentiating without U0126 (blue); untreated Rat CX cells differentiating with 3 μM U0126 (green); and Rat CX cells treated with U0126 for 40 dbs differentiating without U0126 (red). Mean ± s.d., n = 6. *P < 0.05, ns, not significant. (E) A model summarizing the effect of ERKi compounds on fetal NP cells. Erk signaling is a key node regulating the balance between the self-renewal and neuronal differentiation of fetal NP cells. By inhibiting Erk phosphorylation, the Akt pathway is activated via Erk-Akt crosstalk, shifting the balance toward the self-renewing proliferation of fetal NP cells.