Scheme 2.
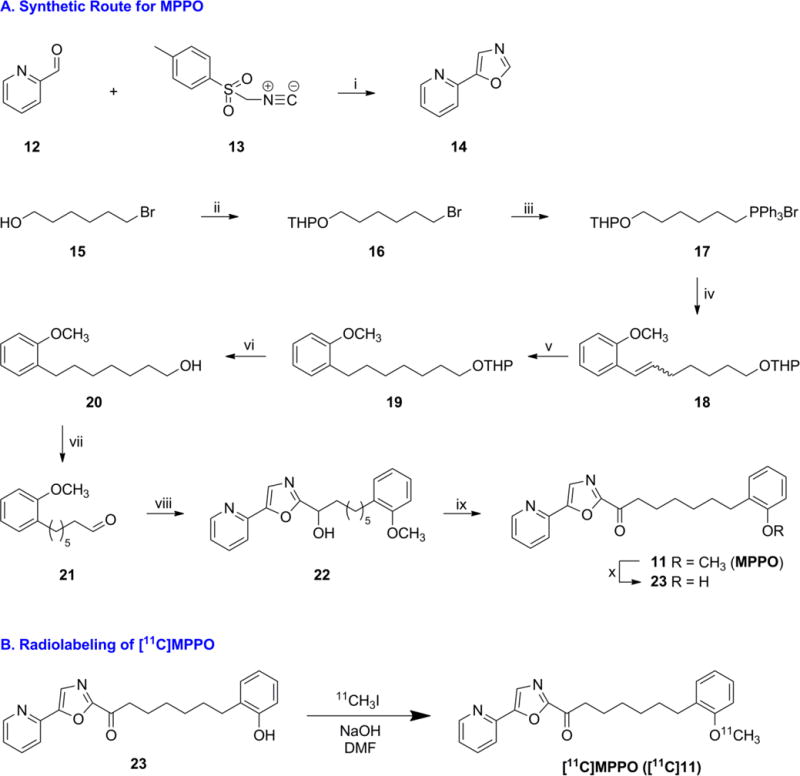
Syntheses of standard and precursor, and radiolabeling of [11C]MPPO
aReagents and conditions: (i) K2CO3, MeOH, 96%. (ii) 3,4-dihydro-2H-pyran, PPTS, CH2Cl2, 85%. (iii) PPh3, 120 °C. (iv) 2-methoxybenzaldehyde, LiHMDS, THF, 56% over two steps. (v) Pd/C, H2, MeOH, 99%. (vi) p-Toluenesulfonic acid monohydrate, MeOH, 89%. (vii) (COCl)2, DMSO, Et3N, CH2Cl2, 83%. (viii) 14, iPrMgCl, THF. (ix) Dess-Martin periodinane, CH2Cl2, 51% over two steps. (x) BBr3, CH2Cl2, 58%.
