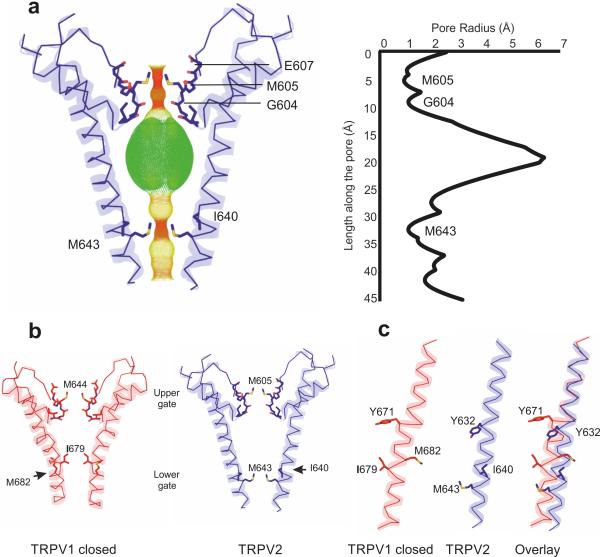
Figure 2.
Pore structure of TRPV2. (a) Profile of the TRPV2 pore generated with HOLE software27 indicates two main constrictions: one at the selectivity filter formed by the side chains of Met605 and the backbone carbonyls of Gly604, and the second one close to the helix-bundle crossing, formed by the side chains of Met643. (b) Comparison of the pores of the closed TRPV1 and the TRPV2 channels, showing that the lower gate of TRPV1 is formed by Ile679, whereas the lower gate in TRPV2 is one turn lower, at Met643. (c) Comparison of the TRPV1 and TRPV2 S6 helices shows that the S6 helix in TRPV1 contains a π-helical segment, whereas the S6 in TRPV2 is α-helical.