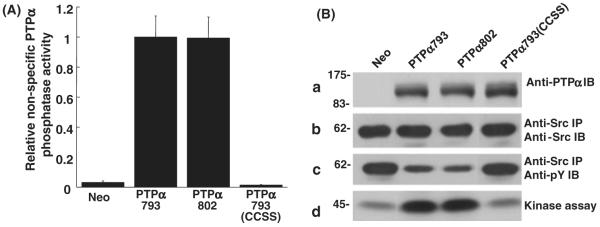
Figure 2.
In vitro dephosphorylation of nonspecific and Src substrates by PTPα. (A) Anti-HA immunoprecipitates from lysates from PTPα793, PTPα802 or PTPα793(CCSS) overexpressor cells (induced by removal of doxycycline for 20 h) were incubated with [32P]phosphotyrosine-containing MBP and incubated for 5 or 10 min (in separate experiments to verify reaction linearity) at 30 °C. The amount of [32P] phosphate released was determined by scintillation counting. Immunoblots were used to determine the amount of PTPα present in each reaction and specific activities (relative to that of PTPα793) and standard errors of the mean (n = 3) were computed. (B) Wild-type Src was immunoprecipitated from Src overexpressor cells and subjected to in vitro dephosphorylation by control (Neo) or PTPα proteins that had been immunopurified from induced overexpressor cells. [Equality of the amounts of immunopurified PTPα proteins added to the phosphatase reactions was verified by immunoblotting with anti-PTPα polyclonal antibody (panel a).] The reaction products were divided into three portions that were immunoblotted with either anti-Src (panel b) or anti-phosphotyrosine (panel c) monoclonal antibodies, or used in an in vitro Src kinase assay with [γ-32P]ATP and acid-denatured enolase as substrate (panel d). Src tyrosine phosphorylation was reduced 51 ± 10%, 56 ± 12% or 9 ± 6% (n = 3) by over-expression of PTPα793, PTPα802 or PTPα793(CCSS), respectively; Src kinase activity was increased by factors of 3.4 ± 0.8, 3.6 ± 1.2 or 1.1 ± 0.1 (n = 3; errors are SEMs) by PTPα793, PTPα802 or PTPα793(CCSS), respectively. The positions of molecular weight markers (in kDa) are indicated.