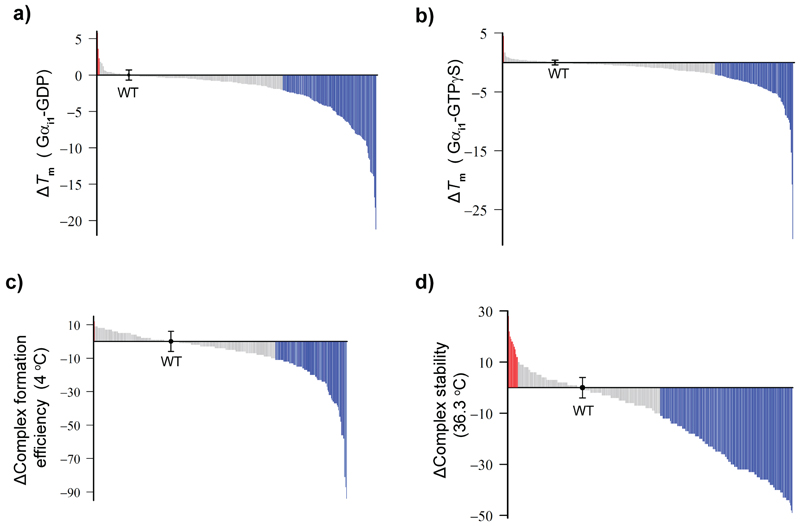
Figure 2. Distribution of effects of Gαi1 alanine mutants on the nucleotide-bound and receptor-bound states.
a, b, Changes in stability (∆Tm) of Gαi1(Ala)-GDP (w/ 1mM GDP) (a) and ∆Tm of Gαi1(Ala)-GTPγS (w/ 0.1mM GTPγS) (b). Gray: -2°C < ∆Tm < 2°C; blue: ∆Tm < -2°C; red: 2°C < ∆Tm. The ∆Tm of Gαi1(WT)-GDP or -GTPγS was shown as the black dot. c, Distribution of ∆ complex formation efficiency of Rho*-Gi(Ala). Blue: ∆ complex formation efficiency is less than -10%; gray: between -10% and 10%; red: more than 10%. d, Distribution of ∆complex stability of Rho*-Gi(Ala). Blue: ∆complex stability is less than -10%; gray: between -10% and 10%; red: more than 10%. The definition of ∆Tm, ∆complex formation efficiency, and ∆complex stability are described in the methods section. All data are presented in Supplementary Table 1. As for ∆Tm of Gαi1 (WT)-GDP and Gαi1(Ala)-GTPγS, data points represent mean ± s.d. from 25 and 24 individual experiments, respectively. As for ∆complex formation efficiency and ∆complex stability of Rho*-Gi(WT), data points represent mean ± s.d. of 33 and 38 individual experiments, respectively.