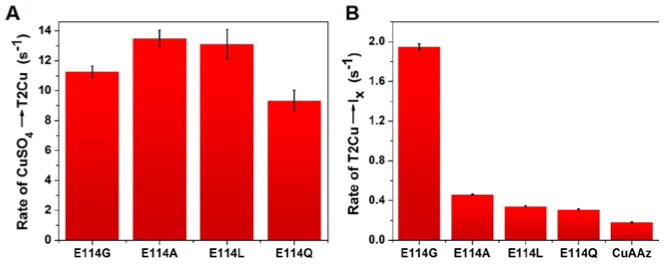
Figure 3.
A) Rates of CuSO4→T2Cu, and B) T2Cu→Ix conversion of CuA variants obtained from the Stopped-flow data. Rates in A) are similar in all variants. In B) highest rate is observed for Glu114GlyCuAAz which supports the hypothesis that the T2Cu→Ix conversion involves a conformation change of the CuA ligand loop as Gly has the most flexible backbone conformation, and thus favors such conformation change compared to the other amino acid side chains.