Abstract
Study Objectives:
Measures of baseline sleep apnea disease burden (apnea-hypopnea index, Epworth Sleepiness Scale) predict continuous positive airway pressure (CPAP) adherence, but composite indices of sleep apnea severity (Sleep Apnea Severity Index, Modified Sleep Apnea Severity Index) may be more robust measures of disease burden. We tested the relative prognostic ability of each measure of sleep apnea disease burden to predict subsequent CPAP adherence and subjective sleep outcomes.
Methods:
Prospective cohort study at a tertiary academic sleep center. Patients (n = 323) underwent initial diagnostic polysomnography for suspected obstructive sleep apnea and 6 mo of subsequent CPAP therapy
Results:
Baseline apnea-hypopnea index and both composite indices predicted adherence to CPAP therapy at 6 mo in multivariate analyses (all p ≤ 0.001). Baseline Epworth Sleepiness Scale did not predict CPAP adherence (p = 0.22). Both composite indices were statistically stronger predictors of CPAP adherence at 6 mo than apnea-hypopnea index (p < 0.001). In multivariate analyses, baseline apnea-hypopnea index (p < 0.05) and both composite indices (both p < 0.04) predicted change in Pittsburgh Sleep Quality Index, whereas only the composite indices predicted changes in Sleep Apnea Quality of Life Index (both p < 0.001). Adjustment for treatment adherence did not affect the relationship of the composite indices with change in Sleep Apnea Quality of Life Index (both p ≤ 0.005).
Conclusions:
Composite indices of baseline sleep apnea severity better predict objective CPAP adherence and subjective treatment outcomes than baseline apnea-hypopnea index and baseline Epworth Sleepiness Scale.
Citation:
Balakrishnan K, James KT, Weaver EM. Predicting CPAP use and treatment outcomes using composite indices of sleep apnea severity. J Clin Sleep Med 2016;12(6):849–854.
Keywords: cohort studies, illness burden, obstructive sleep apnea, patient adherence, prospective studies
INTRODUCTION
Continuous positive airway pressure (CPAP) is first-line therapy for obstructive sleep apnea syndrome. The daily and long-term benefits of CPAP are dependent on patient use of the therapy.1–3 However, up to 50% of patients offered CPAP decline the treatment before initiation or within 1 w of initiation, and up to 25% of patients who accept CPAP discontinue use within 3 y of starting.4 Identifying patients who are at higher risk of CPAP nonadherence may present an opportunity for early intervention with more intense CPAP support. Although individual measures of sleep apnea severity and disease burden (e.g., apnea-hypopnea index [AHI] and Epworth Sleepiness Scale) predict CPAP use and outcome, a multivariable composite measure of sleep apnea severity may be a more robust predictor.
The Sleep Apnea Severity Index (SASI) and the Modified Sleep Apnea Severity Index (modified SASI) are multivariable composite measures of sleep apnea severity.5,6 These indices incorporate polysomnographic (AHI and lowest oxyhemoglobin saturation), subjective (daytime sleepiness), and anthropometric (body mass index [BMI] and presence of redundant pharyngeal mucosa [SASI] or tonsil grade [modified SASI]) measures into a composite severity staging system. These indices have been validated and reflect the breadth of sleep apnea disease burden better than AHI alone.5,6
BRIEF SUMMARY
Current Knowledge/Study Rationale: The daily and long-term benefits of continuous positive airway pressure (CPAP) are dependent on patient use of the therapy. Although individual measures of sleep apnea severity and disease burden (e.g., apneahypopnea index and Epworth Sleepiness Scale) predict CPAP use and outcome, we investigated whether a multivariable composite measure that integrated subjective and objective measures of sleep apnea severity was a more robust predictor.
Study Impact: Composite measures of sleep apnea severity have value in prospectively predicting objective adherence to CPAP treatment and subjective treatment outcomes. They retain the predictive value of apnea-hypopnea index and Epworth Sleepiness Scale for some outcomes while better predicting others, particularly changes in disease-specific quality of life.
In this study, we aimed to evaluate the SASI and modified SASI as multivariable predictors of CPAP use and outcomes. This study tested the hypotheses that (1) the baseline SASI and modified SASI (measured at the time of initial diagnostic polysomnography) would be stronger predictors of CPAP use at 6 mo than would baseline AHI or daytime sleepiness measured by the Epworth Sleepiness Scale, and (2) the baseline SASI and modified SASI would be stronger predictors of changes in subjective sleep quality and sleep apnea-related quality of life with CPAP than would baseline AHI.
METHODS
Study Design
This was a prospective cohort study of adult participants in the Seattle Sleep Cohort at the University of Washington Sleep Center at Harborview Medical Center in Seattle, Washington. Recruitment occurred between August 2006 and February 2008. All participants were recruited on the night of first diagnostic polysomnography. This study was approved by the University of Washington institutional review board.
Participants
Patients were considered eligible if they were at least 18 y old, presented for overnight polysomnography for previously un-diagnosed obstructive sleep apnea, successfully completed polysomnography, and were prescribed CPAP therapy. They also were required to have linguistic and cognitive ability to answer questionnaires and give informed consent. Exclusion criteria included prior diagnosis of sleep apnea, other upper airway obstructive disease, or neurologic, neuromuscular, or pulmonary disease. Patients undergoing either full-night or split-night polysomnography were included in the study.
Data Collection
Baseline data collection was performed by trained research assistants and included targeted history, physical examination, and the following validated questionnaires: Sleep Apnea Quality of Life Index, Short Form-36, Epworth Sleepiness Scale, and Pittsburgh Sleep Quality Index. The specifics of baseline data collection, diagnostic polysomnography, and polysomnography data extraction were as previously described.6 When split-night (combined diagnostic and CPAP titration) polysomnography studies were performed, only data from the diagnostic portion were analyzed for this study.
Follow-Up
Study staff contacted patients by telephone, Email, and postal mail 6 mo after their initial recruitment. At that time, patients were mailed a follow-up questionnaire packet similar to that which they answered at the time of their recruitment, including the Sleep Apnea Quality of Life Index and Pittsburgh Sleep Quality Index. Patients returned the completed questionnaire packet and the CPAP data card by mail.
Questionnaire and physical examination data were entered into an electronic database by trained study staff using a double-entry method to ensure accurate transfer. CPAP use data were extracted directly into an electronic database using a card reader designed for this purpose.
Data Variables
Exposure variables
The AHI was the average number of apneas and hypopneas per hour of sleep as recorded by polysomnography. The Epworth Sleepiness Scale measures subjective sleepiness based on a questionnaire with possible range 0–24. The composite SASI was calculated as previously described based on a combination of subjective (Epworth Sleepiness Scale) and objective (redundant pharyngeal mucosa, body mass index, AHI, lowest oxyhemoglobin concentration) variables.6 The modified SASI is calculated in the same manner but uses dichotomized tonsil size (0–1+, 2–4+) instead of redundant pharyngeal mucosa. Both composite indices have possible values of 1–3 where 3 is most severe sleep apnea severity. AHI and Epworth Sleepiness Scale were also categorized based on commonly used cutoffs (AHI < 15, 15–30, > 30; Epworth < 10, 10–17, > 17) to allow more direct comparison to the three-category scale used by the SASI and modified SASI.
Outcome variables
CPAP use was recorded as mean minutes of use per night averaged over a 4-w period defined as the date 6 mo after diagnostic polysomnography ± 2 w. CPAP acceptance (any use versus no use) was defined as an average of 30 min or more per night. The Sleep Apnea Quality of Life Index and Pittsburgh Sleep Quality Index are questionnaire-based measures that were recorded at the time of diagnostic polysomnography and 6 months later, and differences over that period calculated. The Sleep Apnea Quality of Life Index measures sleep apnea-specific quality of life and has possible score range 1–7 (7 best), whereas the Pittsburgh Sleep Quality Index measures subjective sleep quality and has possible score range 0–21 (21 worst).7–9
Potential confounding variables
Age was measured in years at the time of diagnostic polysomnography. Race was dichotomized as white/nonwhite based on participant self-identification. Annual income was dichotomized at $50,000 based on US census data indicating that median household income in Washington state in 2005 was $49,262 ± 644.10 Smoking was measured as a yes/no binary variable based on participant report.
Analysis
Statistical analysis used Stata/SE 9 software (StataCorp LP, College Station, TX). Bivariate associations between predictors of CPAP use and measured CPAP use were examined using Spearman correlation coefficients. Correlation coefficients were compared using standard errors generated by normal-approximation bootstrapping11 with seed = 12345 and 200 repetitions, chosen a priori. Multivariate analysis controlling for potential confounders was performed using multivariable linear regression for total CPAP use and multivariable logistic regression for CPAP acceptance (any use versus no use).
Bivariate associations between predictors of outcome and changes in Sleep Apnea Quality of Life Index score or Pittsburgh Sleep Quality Index score were examined using analysis of variance, whereas multivariate analysis was performed with multivariable linear regression. Epworth Sleepiness Scale was not tested as a predictor of changes in these subjective outcomes variables because we specifically aimed to compare the standard measure of objective disease severity (AHI) to the SASI and modified SASI for these outcome measures.
Several secondary analyses were performed. Spearman correlation coefficients were used to examine individual components of the SASI and modified SASI (apart from AHI and the Epworth Sleepiness Scale) for associations with CPAP use. The exposure variables were also tested for prediction of CPAP acceptance (use versus no use) using simple and multivariable logistic regression. CPAP use in minutes per night was also analyzed as a possible confounder in multivariable analysis to determine whether it affected the relationship between exposure variables and subjective outcome variables.12 p values less than 0.05 were considered statistically significant.
RESULTS
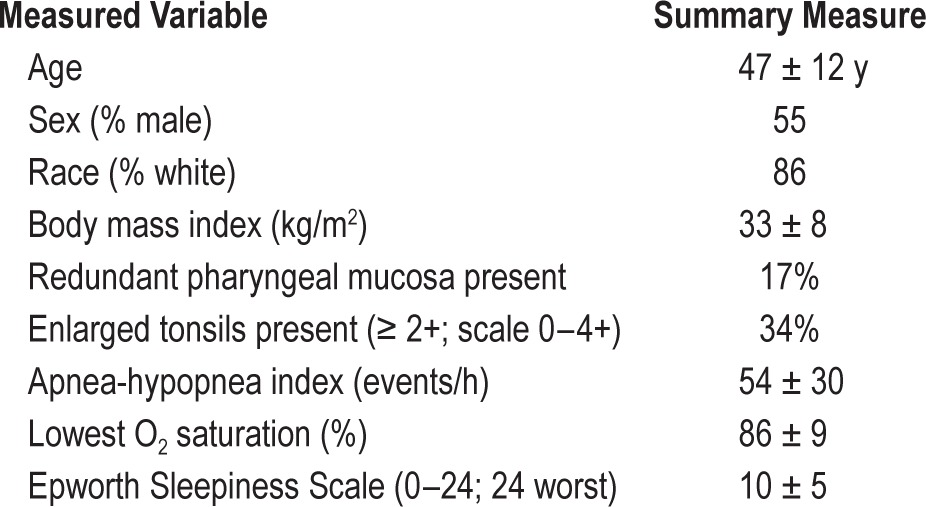
This study included 323 research participants. Descriptive data for the sample are shown in Table 1. Average CPAP use over the 4-w period analyzed varied widely between patients, ranging 0–474 min/night with a sample mean of 132 ± 162 min/ night; 67 patients had no CPAP use at all.
Table 1.
Study sample description.
Bivariate associations between CPAP use and the predictors under study are shown in Table 2. Baseline AHI, SASI, and modified SASI were all significantly correlated with CPAP use at 6 mo, whereas daytime sleepiness as measured by the Epworth Sleepiness Scale was not. The correlation coefficients for SASI and modified SASI were not significantly different (p = 0.21); both indices were better correlated with CPAP use than were AHI or Epworth Sleepiness Scale (p < 0.001).
Table 2.
Estimated Spearman correlation coefficients reflecting associations between predictors of continuous positive airway pressure use (measured at the time of diagnosis) and continuous positive airway pressure use.

In multivariable regression adjusting for age, sex, race, income, and smoking history, baseline AHI (p = 0.001), SASI (p < 0.001), and modified SASI (p < 0.001) were significant predictors of CPAP use, whereas the Epworth Sleepiness Scale was not (p = 0.21). Specific linear regression coefficients are not listed here because AHI, SASI, modified SASI, and Ep-worth Sleepiness Scale each have different scales, preventing useful comparison of coefficients.
Secondary analyses further clarified these associations. Lowest oxyhemoglobin saturation was significantly correlated with CPAP use (r = −0.21; p = 0.01) whereas pharyngeal mu -cosal redundancy (r = 0.002; p = 0.98), tonsil size (r = −0.06; p = 0.54), and BMI (r = 0.14; p = 0.10) were not.
Another secondary analysis examined prediction of CPAP acceptance. Bivariate analysis with simple logistic regression indicated that increasing values of baseline AHI (odds ratio [OR] = 1.02; 95% confidence interval [CI] 1.01, 1.03; p = 0.002), SASI (OR = 2.1; 95% CI 1.6, 2.6; p = 0.002), and modified SASI (OR = 2.2; 95% CI 1.7, 2.7; p = 0.001) significantly predicted acceptance, whereas Epworth Sleepiness Scale (OR = 1.05; 95% CI 1.01, 1.09; p = 0.14) did not. Similarly, categorized AHI predicted CPAP acceptance (OR = 2.8; 95% CI 1.8, 3.9; p = 0.006), whereas the categorized Epworth Sleepiness Scale did not (OR = 1.31; 95% CI 0.99, 1.63; p = 0.271).
Multivariable logistic regression adjusting for age, sex, race, income, and smoking history revealed a similar pattern. AHI (OR = 1.02; 95% CI 1.01, 1.03; p = 0.003), categorized AHI (OR = 2.9; 95% CI 1.7, 4.0; p = 0.008), SASI (OR = 2.1; 95% CI 1.6, 2.6; p = 0.003), and modified SASI (OR = 2.2; 95% CI 1.7, 2.8; p = 0.001) significantly predicted CPAP use after adjusting for all of these potential confounders. Epworth Sleepiness Scale (OR = 1.05; 95% CI 1.01, 1.09; p = 0.224) and catego -rized Epworth Sleepiness Scale (OR = 1.20; 95% CI 0.89, 1.51; p = 0.481) did not.
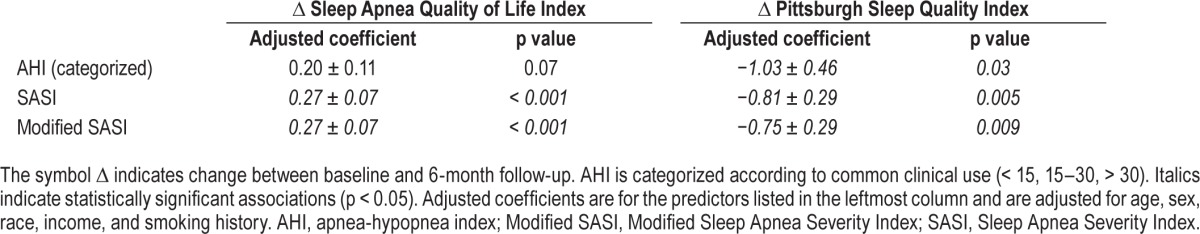
In the bivariate associations between AHI, SASI, modified SASI, and 6-mo changes in Sleep Apnea Quality of Life Index and Pittsburgh Sleep Quality Index, all three predictors were significantly associated with change in subjective sleep quality (p = 0.045, p = 0.01, and p = 0.04, respectively) but only SASI and modified SASI were associated with change in sleep apneaspecific quality of life (p = 0.23, p = 0.001, and p = 0.001, re -spectively). For this analysis, AHI was categorized as described earlier. After adjustment for age, sex, race, income, and smoking history, SASI and modified SASI remained strongly signifi-cant predictors of change in sleep apnea-specific quality of life (p < 0.001), whereas AHI showed a trend toward significance (p = 0.07) (Table 3). All associations with change in sleep quality remained significant after adjustment (Table 3).
Table 3.
Associations between predictors (measured at the time of diagnosis) and subjective outcomes (measured as change over first 6 months after diagnosis).
Multivariate analysis was repeated with mean CPAP use in minutes per night as an additional covariate. SASI (p = 0.005) and modified SASI (p = 0.002) remained significant predictors of change in sleep apnea-specific quality of life, whereas AHI remained non-significant (p = 0.46). In contrast, the associations observed between SASI, modified SASI, and change in sleep quality disappeared after adjustment for CPAP use (SASI p = 0.21, modified SASI p = 0.35), whereas AHI remained nonsignificant (p = 0.39).
DISCUSSION
Although CPAP is first-line therapy for adult obstructive sleep apnea, its benefits depend on adequate and proper use by the patient. Accordingly, many predictors of CPAP use have been evaluated in an effort to improve clinicians' ability to discern likely nonusers early, so that targeted preemptive adherence strategies or other therapeutic options may be considered before the patient spends time on a prolonged unsuccessful trial of CPAP. Thus far, no single predictor has demonstrated consistent value, indicating that the SASI and modified SASI might be useful because they may combine the advantages of several single predictors.
Because CPAP use is a surrogate measure, many variables are also used to anticipate the clinical effects of CPAP therapy on important symptoms such as sleep quality and quality of life. Again, no single baseline measure clearly predicts these outcomes of interest, further clouding the clinician's ability to identify patients who might benefit most from CPAP.13–15
In this study, both baseline SASI and modified SASI were statistically and clinically stronger predictors of objectively measured nightly CPAP use at 6 mo than were either AHI or baseline Epworth Sleepiness Scale score. Although both composite indices and AHI were significantly associated with quantitative CPAP use after adjustment for multiple potential confounders, the composite indices were still statistically significantly more strongly associated. This finding suggests that these indices both preserve and improve on AHI's predictive value for CPAP use.
This possibility was explored with analysis of the individual components of the SASI and modified SASI. We found that although AHI makes up a portion of the indices' predictive value, the inclusion of lowest oxyhemoglobin saturation and BMI are likely also important. Lowest oxyhemoglobin saturation was negatively associated with CPAP use, indicating that lower saturations predicted increased use. Similarly, BMI showed a statistical trend of association with CPAP use. These associations may reflect a feedback process in which patients with greater sleep apnea disease burden experience greater improvements with CPAP use, leading to more CPAP use. Although this adequately powered study found no significant predictive value for daytime sleepiness alone, this variable may interact with other components of the SASI or modified SASI and strengthen their predictive value. The inclusion of daytime sleepiness in the form of the Epworth Sleepiness Scale score does not appear to detract from the prognostic value of the indices, as they are still statistically more strongly predictive than either AHI of Epworth Sleepiness Scale score alone.
The secondary analysis examining CPAP acceptance is of interest because other authors have indicated that up to a quarter of patients who begin CPAP therapy discontinue it within 3 y of starting.4 If these patients were detected at the time of diagnosis, their adherence to CPAP might benefit from targeted counseling, troubleshooting, and close follow-up initiated early in treatment. Our analysis demonstrates that AHI has predictive value for acceptance, and the composite indices retain that value. Given that the composite indices also predict quantitative CPAP use more precisely than AHI, they may have greater value as tools for predicting future treatment adherence.
The SASI and modified SASI predicted 6-mo change in sleep apnea-specific quality of life, whereas AHI did not. These associations persisted after further adjustment for CPAP use, indicating that the composite indices have predictive value for this important symptomatic outcome beyond the effect of CPAP use on quality of life. Indeed, the regression coefficients for the indices changed very little when adjusted for CPAP use, suggesting that this value may be entirely separate from that of CPAP use.
AHI, SASI, and modified SASI all significantly predicted 6-mo change in subjective sleep quality. When adjusted for several potential confounders, the SASI and modified SASI retained this association, while AHI did not. However, when further adjusted for CPAP use, the composite indices also ceased to be associated with change in sleep quality. This finding suggests that although the SASI and modified SASI have greater value than AHI in predicting improvements in sleep quality early in treatment, this value is largely due to their predictive value for quantitative CPAP use. It is not surprising that associations with sleep quality are less robust than those with sleep apnea-specific quality of life, as the former measure is not disease-specific and thus is vulnerable to the effects of more factors. For example, the Pittsburgh Sleep Quality Index takes into account sleep times and durations, which may be affected by multiple factors in the patient's environment. The Sleep Apnea Quality of Life Index, meanwhile, is specific to problems related to sleep apnea itself.
Strengths and Limitations
This study used an adequately powered prospective cohort design. Accordingly, its conclusions about predictive value for CPAP use and subjective outcomes are credible because of the temporal relationship between predictors and outcomes and of the strength of associations. However, as with any controlled observational study design, the predictive associations are vulnerable to confounding. While we adjusted for known important confounders, there are other potential confounding variables, both known and unknown.
Two other limitations pertain to AHI, SASI, and modified SASI. First, all three measures are incomplete measures of obstruction severity. For example, each relies on the frequency of apneas and hypopneas without accounting for the duration of these breathing events. The composite indices do incorporate measures of associated pathophysiologic features (desaturation) and presumed clinical effect of sleep apnea (sleepiness). Further research is necessary to address ways of measuring and incorporating more comprehensive and meaningful patho-physiological features of sleep apnea into the assessment of these patients. This limitation is inherent to the current standards of sleep testing and is not specific to this study.
The second limitation, again common to all three severity measures, is the failure to consider individual personality, emotional, and mental health characteristics that may affect the relationship between objective and subjective disease burden, such as depression and hypochondriasis.16,17 The differential clinical susceptibility to the pathophysiologic processes of sleep apnea is an inherent limitation of physiologic surrogates. By incorporating a broader measure of sleep apnea disease burden and incorporating clinical effects of sleep apnea (sleepiness), the composite indices are likely less susceptible to the differential susceptibility. Furthermore, sleep apnea that is characterized by excessive daytime sleepiness may be more responsive to CPAP therapy for a variety of outcomes (e.g., incident hypertension18), so it is an advantage that this susceptibility factor is incorporated in the SASI and modified SASI.
Generalizability
Our study sample appears to be similar to the general sleep apnea population; a recent random telephone survey matched to US-wide regional age distributions found that men were generally at higher risk than women, ages 50–64 y were at highest risk, and risk rose markedly with BMI ≥ 30.19 However, our predominantly Caucasian sample may not reflect sleep apnea prevalence patterns in other racial groups.20,21
CONCLUSIONS
This study demonstrates that the previously described composite SASI and modified SASI have value in prospectively predicting objective adherence to CPAP treatment and subjective treatment outcomes, particularly changes in disease-specific quality of life, more than AHI or Epworth Sleepiness Scale alone. These findings are important because patients seek care due to poor quality of life and symptom burden, not because they are concerned about their rate of apneas and hypopneas per se. Further study will be needed to confirm these findings and to address the limitations of this work. Until those studies happen, the SASI and modified SASI appear to be useful and potentially important tools for clinicians and researchers interested in this disease.
DISCLOSURE STATEMENT
This was not an industry supported study. Supported by an AAO-HNSF Health Services Research Grant (KB), NIH F32 HL090226 (KB), NIH T32 DC000018 (KB, EMW), NIH K23 HL068849 (EMW), and NIH R01 HL084139 (EMW). Dr. Weaver is partially supported by resources from the Veterans Affairs Puget Sound Healthcare System, Seattle, WA.The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. The authors have indicated no financial conflicts of interest. This work was performed at the University of Washington and Harborview Medical Center, Seattle, WA.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- Modified SASI
Modified Sleep Apnea Severity Index
- SASI
Sleep Apnea Severity Index
REFERENCES
- 1.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–9. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campos-Rodriguez F, Pena-Grinan N, Reyes-Nunez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest. 2005;128:624–33. doi: 10.1378/chest.128.2.624. [DOI] [PubMed] [Google Scholar]
- 3.Chin K, Nakamura T, Takahashi K, et al. Falls in blood pressure in patients with obstructive sleep apnoea after long-term nasal continuous positive airway pressure treatment. J Hypertens. 2006;24:2091–9. doi: 10.1097/01.hjh.0000244960.69985.4c. [DOI] [PubMed] [Google Scholar]
- 4.Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS) Sleep Med Rev. 2003;7:81–99. doi: 10.1053/smrv.2001.0197. [DOI] [PubMed] [Google Scholar]
- 5.Piccirillo JF, Gates GA, White DL, Schechtman KB. Obstructive sleep apnea treatment outcomes pilot study. Otolaryngol Head Neck Surg. 1998;118:833–44. doi: 10.1016/S0194-5998(98)70277-3. [DOI] [PubMed] [Google Scholar]
- 6.Balakrishnan K, James KT, Weaver EM. Composite severity indices reflect sleep apnea disease burden more comprehensively than the apnea-hypopnea index. Otolaryngol Head Neck Surg. 2013;148:324–30. doi: 10.1177/0194599812464468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flemons WW, Reimer MA. Development of a disease-specific health-related quality of life questionnaire for sleep apnea. Am J Respir Crit Care Med. 1998;158:494–503. doi: 10.1164/ajrccm.158.2.9712036. [DOI] [PubMed] [Google Scholar]
- 8.Flemons WW, Reimer MA. Measurement properties of the Calgary Sleep Apnea Quality of Life Index. Am J Respir Crit Care Med. 2002;165:159–64. doi: 10.1164/ajrccm.165.2.2010008. [DOI] [PubMed] [Google Scholar]
- 9.Buysse DJ, Reynolds CF, 3rd, Mon TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Census Bureau. [Accessed July 15, 2014]. http://factfinder2.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_05_EST_S1903&prodType=table.
- 11.Haukoos JS, Lewis RJ. Advanced statistics: bootstrapping confidence intervals for statistics with “difficult” distributions. Acad Emerg Med. 2005;12:360–5. doi: 10.1197/j.aem.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Kirkham EM, Weaver EM. Relationship between clinical and polysomnography measures corrected for CPAP use. J Clin Sleep Med. 2015;11:1305–12. doi: 10.5664/jcsm.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weaver EM, Kapur V, Yueh B. Polysomnography vs. self-reported measures in patients with sleep apnea. Arch Otolaryngol Head Neck Surg. 2004;130:453–8. doi: 10.1001/archotol.130.4.453. [DOI] [PubMed] [Google Scholar]
- 14.Weaver EM, Woodson BT, Steward DL. Polysomnography indexes are discordant with quality of life, symptoms, and reaction times in sleep apnea patients. Otolaryngol Head Neck Surg. 2005;132:255–62. doi: 10.1016/j.otohns.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;3:CD001106. doi: 10.1002/14651858.CD001106.pub3. [DOI] [PubMed] [Google Scholar]
- 16.Hayashida K, Inoue Y, Chiba S, et al. Factors influencing subjective sleepiness in patients with obstructive sleep apnea syndrome. Psychiatry Clin Neurosci. 2007;61:558–63. doi: 10.1111/j.1440-1819.2007.01707.x. [DOI] [PubMed] [Google Scholar]
- 17.Bardwell WA, Moore P, Ancoli-Israel S, Dimsdale JE. Fatigue in obstructive sleep apnea: driven by depressive symptoms instead of apnea severity? Am J Psychiatry. 2003;160:350–5. doi: 10.1176/appi.ajp.160.2.350. [DOI] [PubMed] [Google Scholar]
- 18.Kapur VK, Weaver EM. Filling in the pieces of the sleep apnea-hypertension puzzle. JAMA. 2012;307:2197–8. doi: 10.1001/jama.2012.5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiestand DM, Britz P, Goldman M, Phillips B. Prevalence of symptoms and risk of sleep apnea in the US population: results from the National Sleep Foundation Sleep in America 2005 poll. Chest. 2006;130:780–6. doi: 10.1378/chest.130.3.780. [DOI] [PubMed] [Google Scholar]
- 20.Arias MA, Alonso-Fernandez A, Garcia-Rio F. Obstructive sleep apnea in minorities. Am J Med. 2007;120:e17. doi: 10.1016/j.amjmed.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 21.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]


