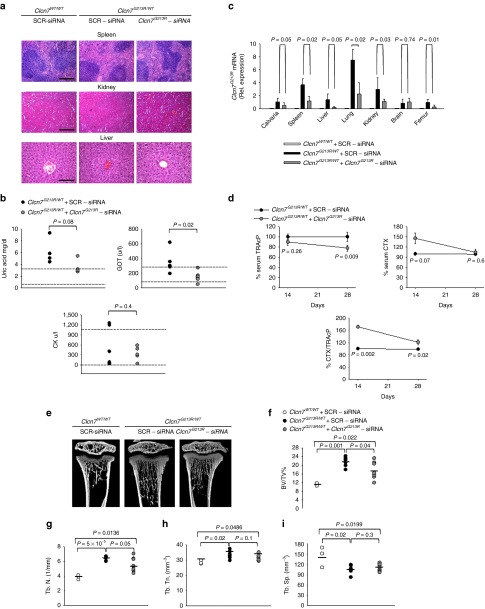
Figure 2.
In vivo treatment and safety study. Ten-day-old Clcn7G213R/WT mice were injected i.p. with 4 mg/kg of Clcn7G213R-sticky siRNA jetPEI conjugate, three times a week for 4 weeks. At the end of the experiments, mice were sacrificed and (a) the indicated organs were subjected to histopathological evaluation by hematoxylin/eosin staining (Bar = 100 µm for spleen and kidney, 20 µm for liver). (b) Sera were collected and analyzed by the Reflotron method for the indicated biomarkers of kidney and liver disease, and for the ADO2 biomarker CK. Normal values are between the two dotted lines. (c) RNA was extracted from the indicated organs and subjected to real time RT-PCR using primer pairs specific for the Clcn7G213R mRNA, normalized for gapdh. (d) Ten day-old Clcn7WT/WT and Clcn7G213R/WT were treated with 4 mg/kg of scrambled- (SRC) or Clcn7G213R-sticky siRNA jetPEI conjugate, three times a week for 2 and 4 weeks. At the end of the experiments, mice were sacrificed, then the serum biomarker of bone resorption, CTX, the serum osteoclast biomarker, TRAcP (5b isoform), and the CTX/TRAcP ratio were evaluated after 2 and 4 weeks of treatment. (e) µCT analysis of proximal tibias of mice treated with 4 mg/kg of Clcn7G213R-sticky siRNA jetPEI conjugate, three times a week for 2 weeks, followed by measurements of trabecular (f) bone volume over total tissue volume (BV/TV), (g) trabecular number (Th.N), (h) thickness (Tb.Th), and (i) separation (Tb.Sp). Data are (a,e) representative or (b–d, f–i) the mean ± SD of four to seven mice per group (Student's t-test). For f–i statistics was also performed by one-way analysis of variance (shown in Supplementary Table S3).