Fig. 2.
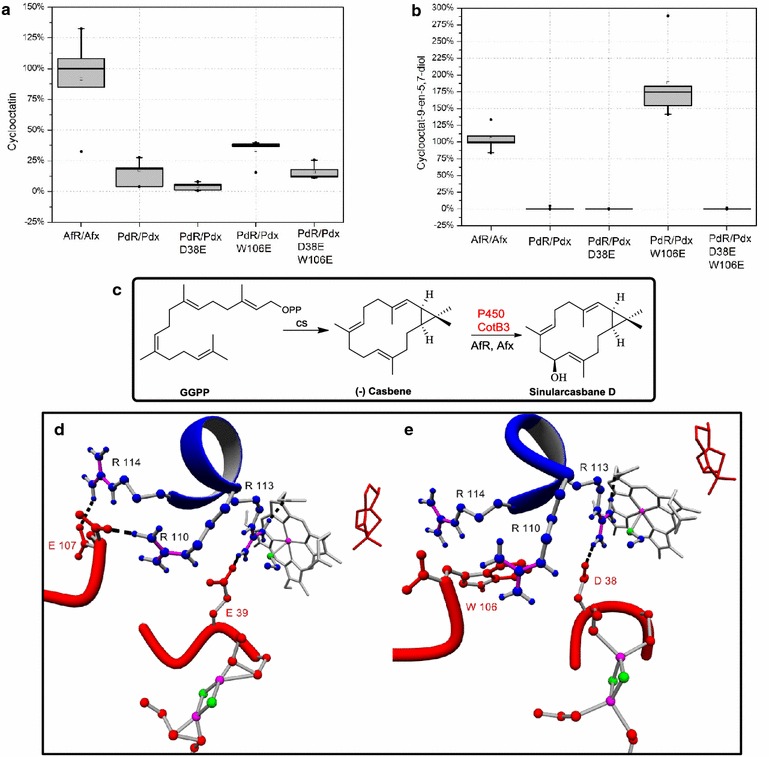
Redox partner induced CotB3/CotB4 substrate promiscuity in a designed E.coli production system. a, b Whole cell hydroxylation experiments using E. coli harboring either a CotB3 or b CotB4 combined with different wild type and mutant redox partners. a Cycloocat-9-en-7-ol or b cyclooctat-9-en-5,7-diol have been added in vitro. c Biocatalytic pathway from GGPP to (−)−casbene by the casbene synthase for Jatropha curcas (CS) and subsequent hydroxylation to sinularcasbane D by CotB3. d Enlargement of the Cotb3·Afx complex depicting essential molecular interactions. Afx (red) contains inorganic Fe2/S2-cluster (green/magenta). CotB3 (blue) contains prosthetic heme group (gray; Fe, magenta) and cyclooct-9-en-7-ol (red). Hydrogen bonds essential for the successful binding and electron transfer between Afx and CotB3 are shown in black. e Enlargement of the CotB3·Pdx complex depicting essential molecular interactions. Pdx (red) contains inorganic Fe2/S2-cluster (green/magenta). CotB3 (blue) contains prosthetic heme group (gray; Fe, magenta) and cyclooct-9-en-7-ol (red). Hydrogen bonds essential for the successful binding and electron transfer between Afx and CotB3 are shown in black
