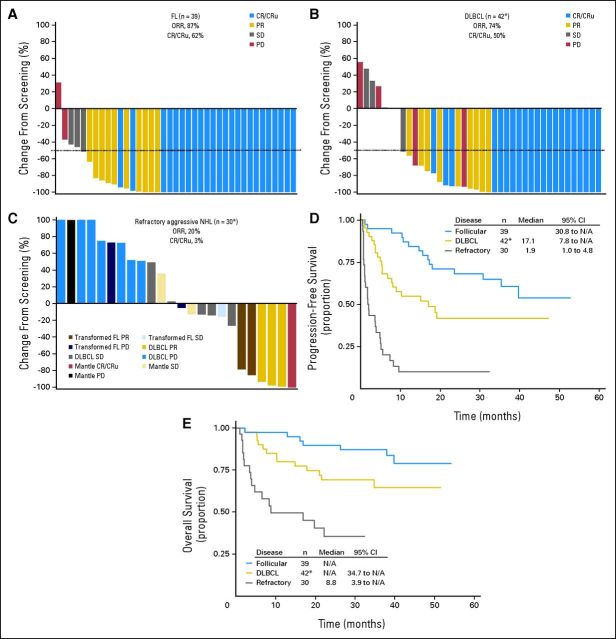
Fig 3.
In this study, tumor assessments were performed at screening after every two cycles and at end-of-treatment visit. Tumor assessments continued after end-of-treatment visit every 12 weeks until progression, death, or administration of another anticancer therapy, whichever occurred first. After progression or new therapy, patients were observed for survival for up to 5 years. (A-C) Waterfall plots for patients enrolled to receive maximum-tolerated dose (MTD) treatment by best investigator-reported objective response. Nodal lesion sizes were normalized for normal nodal structures (defined as 100 mm2 for nodal lesions with maximum diameter < 15 mm at baseline and 150 mm2 for those with diameters > 15 mm at baseline14). Objective response rate (ORR) is defined as complete response (CR) plus unconfirmed complete response (CRu) plus partial response (PR). (A) ORR and CR/CRu rates and change in lesion size for all relapsed patients with follicular lymphoma (FL) enrolled to receive MTD treatment (n = 39); (B) ORR and CR/CRu rates for all relapsed patients with diffuse large B-cell lymphoma (DLBCL) enrolled to receive MTD treatment (n = 42), with change in lesion size shown for 40 patients. (*) Not shown for two patients: one because of incomplete radiographic tumor assessment at screening, and one, who did not receive inotuzumab ozogamicin [INO], because of no postscreening radiographic tumor assessment); (C) ORR and CR/CRu rates for all patients with refractory aggressive non-Hodgkin lymphoma (NHL) enrolled to receive MTD treatment (n = 30), with change in lesion size shown for 24 patients. (*) Not shown for six patients [five DLBCL, one mantle cell] because there was no or incomplete postscreening radiographic tumor assessment). Kaplan-Meier curves of (D) progression-free survival; (*) includes one patient who did not receive INO. (E) Overall survival for all patients enrolled to receive MTD treatment by NHL type; (*) includes one patient who did not receive INO. N/A, not applicable; PD, progressive disease; SD, stable disease.