Fig. 1.
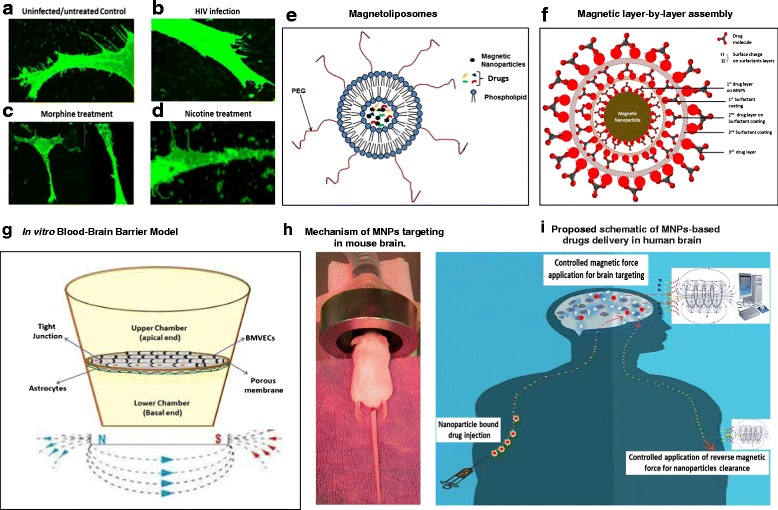
a–d- Confocal microscopy image showing changes in dendritic and spinal morphology of uninfected/untreated (a), HIV infected (b) [64], Morphine (c) [76], and Nicotine treated (d) [62] neuroblastoma (SKNMC) cells; e-f- Types of magnetic nanoformulations: Magnetoliposomes (e) [11] and Magnetic layer-by-layer assembly (f) [72]; g- In vitro BBB model for : Astrocytes-Endothelial cells co-culture in vitro BBB model: Culture plate is bi-compartmentalized via a transwell porous membrane. The top and underside of this membrane is cultured respectively with tightly junctioned endothelial cells and astrocytes which correspondingly mimics the external (peripheral blood side) and internal (brain microenvironment side) surface of BBB. Magnetic force is applied at the bottom of transwell which influence the transmigration of magnetic nanoformulations [11] (h)- Mechanism of MNPs targeting in rodent model: Anesthetized mouse can be placed in a platform with their head positioned between the poles of magnetic coil and retained in the desired field for desired time period. i- Proposed schematic of MNPs-based drugs delivery in human brain: Under the influence of in silico-controlled, non-invasive magnetic force from exterior, drug loaded magnetic nanocarriers can be directly transported across the BBB. Drug release at target is mediated by manually uncontrollable, cellular responses such as change in temperature, pH, intracellular Ca2 + level, etc. or by externally controlled mechanism such as magneto-electric force, radio-frequency magnetic force, etc. Leftover MNPs biodegrades automatically in 2–3 weeks without negative physiological implications in brain or may be cleared immediately by applying reverse magnetic force
