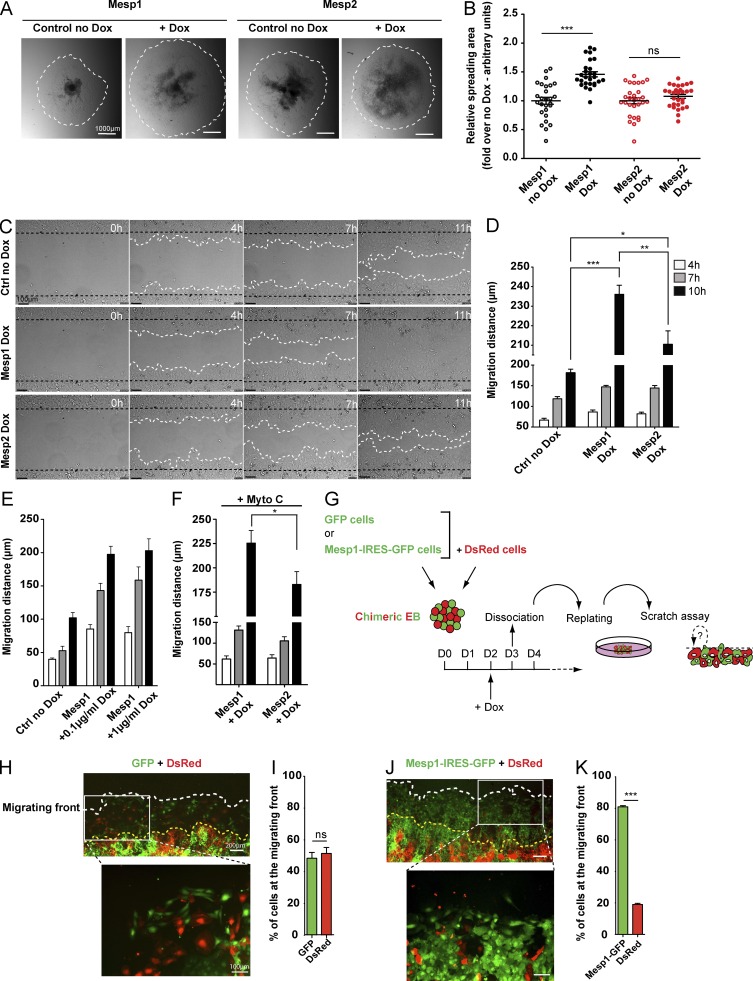
Figure 2.
Mesp1 promotes rapid cell migration by a cell-autonomous mechanism. (A and B) Mesp1- and Mesp2-inducible EBs were replated on a gelatin-coated plate at day 3, 24 h after Dox induction, and the cell spreading areas were measured by bright field microscopy 48 h later. Data represent the relative spreading area of EB ± SEM of at least 27 EBs from four biologically independent experiments. (C) Cell migration measured by in vitro scratch wound assay. Time-lapse microscopy images were recorded every 5 min during 11 h. Data show cell migration 0, 4, 7, and 11 h after the wound. (D) Quantification of the distance of cell migration 4, 7, and 10 h after wounding. Data represent the mean migration distance ± SEM of six biologically independent experiments. Control (no Dox) in C and D represent Mesp1 cells without Dox. No difference was observed between Mesp1 and Mesp2 no Dox cells. (E) Migration distance of control, Mesp1 0.1 µg/ml, and Mesp1 1 µg/ml after 4, 7, and 10 h after Dox addition (n = 3 independent experiments performed in duplicate). (F) Migration distance of Mesp1- and Mesp2-expressing cells after mitomycin C (Mito) treatment (n = 3). (G) Experimental strategy to assess the cell-autonomous function of Mesp1 in the promotion of cell migration. Chimeric EBs were generated by aggregating similar numbers of Mesp1-IRES-GFP–expressing and control DsRed-expressing cells. (H–K) Fluorescence microscopy analysis and quantification of the relative chimerism of GFP- and DsRed-positive cells at the migrating front, 24 h after wounding in chimeric EBs containing control GFP (H and I) or Mesp1-IRES-GFP (J and K) cell lines. Graphs (I and K) represent the mean chimerism and SEM of three independent experiments. At least 150 cells for each condition were counted. Bars: (A) 1,000 µm; (C) 100 µm; (H and J, lower magnification) 200 µm; (H and J, higher magnification) 100 µm. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant.