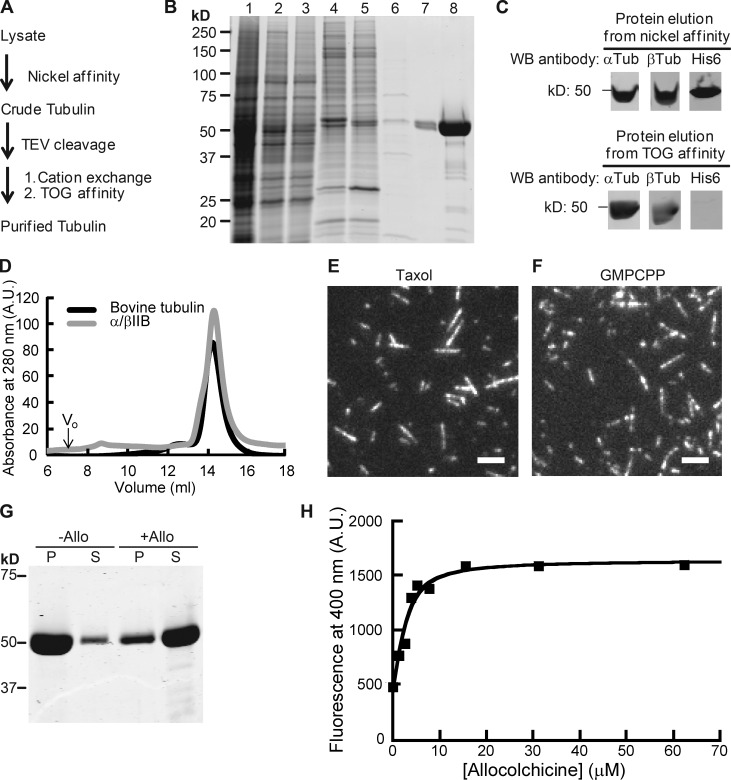
Figure 1.
Purification of recombinant α/βIIB tubulin heterodimers. (A) Purification scheme. (B) SDS-PAGE analysis (1, lysate; 2, supernatant; 3 and 4, nickel affinity: flow-through (3); elution (4); 5, TEV-digested protein elution from nickel affinity column (a band corresponding to the added TEV protein is at ∼25 kD); 6–8, TOG affinity: flow-through (6); elution (7), 20× amount in lane 7 (8); Coomassie stain). (C) Western blot (WB) analyses. Full blots are provided in Fig. S1 A. (D) Elution profiles from size-exclusion chromatography. Peak volume: 14.4 ml (α/βIIB); 14.3 ml (bovine tubulin, used as reference). Void volume (V0) is 7 ml. A.U., arbitrary units. (E and F) TIRF images of taxol-stabilized (E) or GMPCPP (F) microtubules. Bars, 3 µm. (G) SDS-PAGE analysis of tubulin sedimentation in the presence of allocolchicine (+Allo) or 3% DMSO control (−Allo). Pellet (P) and supernatant (S) fractions are indicated. (H) Representative equilibrium binding curve for α/βIIB with allocolchicine from one experiment is shown. Kd = 1.8 ± 0.42 µM (n = 3, mean ± SD). Fig. S1 C shows data averaged from all three experiments and fitted to a single curve.