Figure 1.
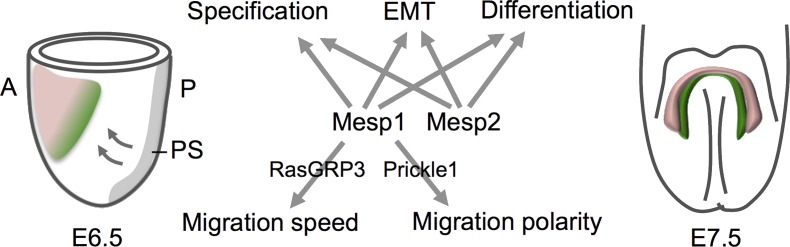
Differential regulation of Rasgrp3 and Prickle1 by Mesp1 and Mesp2 controls migration speed and directionality of nascent cardiac mesoderm. A lateral view of an embryonic day (E) 6.5 mouse embryo (left), showing how cells that transiently express Mesp1 in the primitive streak (PS) migrate (arrows) to form cranial and cardiogenic mesoderm (denoted by the pink and green shaded area). A ventral view of an E7.5 embryo (right) shows differentiated cardiomyocytes in the cardiac crescent (pink), whereas second heart field cells (green), derived from later Mesp1-expressing progenitor cells, retain progenitor cell status and progressively contribute to the elongating heart tube. Mesp1 and Mesp2 are both required for cardiovascular progenitor cell specification, EMT, and differentiation. Chiapparo et al. (2016) now identify Mesp1-specific targets (Prickle and RasGRP3) that regulate the speed and polarity of cardiovascular progenitor cell migration in differentiating ES cells. A, anterior; P, posterior.