Table 1.
Generation of Peptidoglycan Derivatives from 2-Amino-MDP (3)
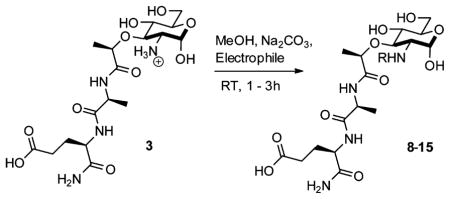
| |||
|---|---|---|---|
| Entry | Electrophile | Product | Yield |
| 1a,b | Acetoxyacetic acid NHS ester | 8,R=C(O)CH2OAc | 48% |
| 2 | Methoxyacetic anhydride | 9,R = C(O)CH2OMe | 78% |
| 3 | Ethyl triflouroacetate | 10,R=C(O)CF3 | 92% |
| 4c | Succinic anhydride | 11,R = C(O)(CH2)2CO2H | 66% |
| 5d | Levulinic acid NHS ester | 12, R = C(O)(CH2)2C(O)Me | 77% |
| 6d | 2-Azidoacetic acid NHS ester | 13,R=C(O)CH2N3 | 88% |
| 7c,e | Dansyl chloride | 14, R= Dansyl | 48% |
| 8 | Biotin NHS ester | 15, R = Biotin | 79% |
Electrophile prepared according to literature precedent.15
Per-formed in H2O with NaHCO3.
Stirred 24 h.
Electrophile prepared with EDC/NHS.
Performed in H2O/DMF.
