Fig. 6.
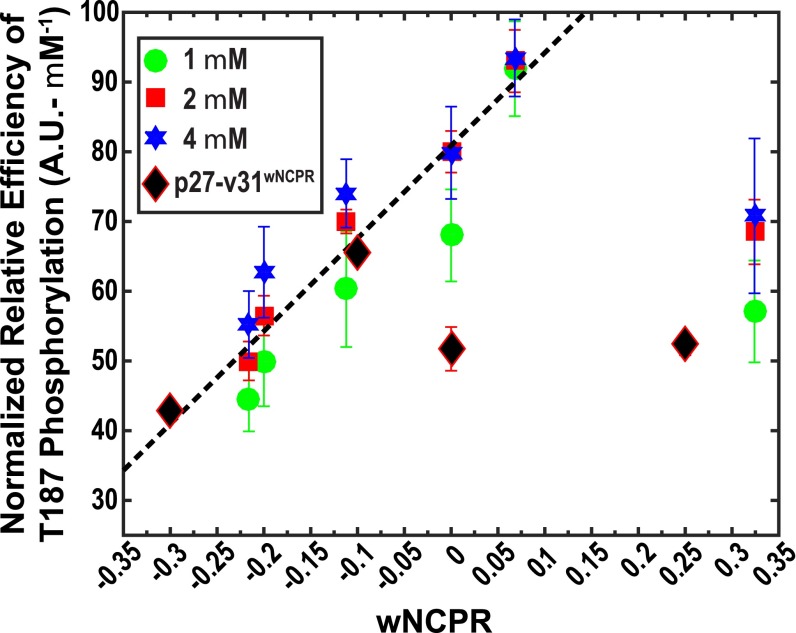
Normalized relative efficiency of T187 phosphorylation plotted against the wNCPR values within auxiliary motifs for p27-vXY and p27-v31(wNCPR) variants. For p27-vXY variants, the datasets correspond to concentrations of 1, 2, and 4 μM of the 1:1:1 ternary complex of p27-vXY/Cdk2/cyclin A. For each dataset, shown using different colors, we calculated the normalized relative efficiency e by dividing the relative efficiencies by the corresponding concentrations of the ternary complex. Within error, the normalized relative efficiencies are coincident for each p27-vXY variant. The Pearson r value quantifying the positive correlation between e and wNCPR is 0.93. For the linear regression and correlation analysis we left out the data for p27-v78. The values along the ordinate can be written as e = m(wNPCR) + c. Here, m is the slope and c is the intercept. Because wNCPR is a dimensionless quantity the slope quantifies the increase in the normalized efficiency per unit rise in wNCPR and the intercept quantifies the normalized relative efficiency if the wNCPR is zero. The values for m and c are 133.1 A.U.⋅μM–1 and 80.9 A.U.⋅μM–1, respectively. This model predicts the normalized relative efficiency of T187 phosphorylation as a function of the wNCPR within the auxiliary motif of p27-vXY. The solid diamonds are data for p27-v31(–0.30), p27-v31(–0.10), p27-v31(0.00), and p27-v31(+0.25), respectively, and test the generality of the linear model in sequences where the κ value is fixed and wNCPR is varied by changes to the NCPR profile.