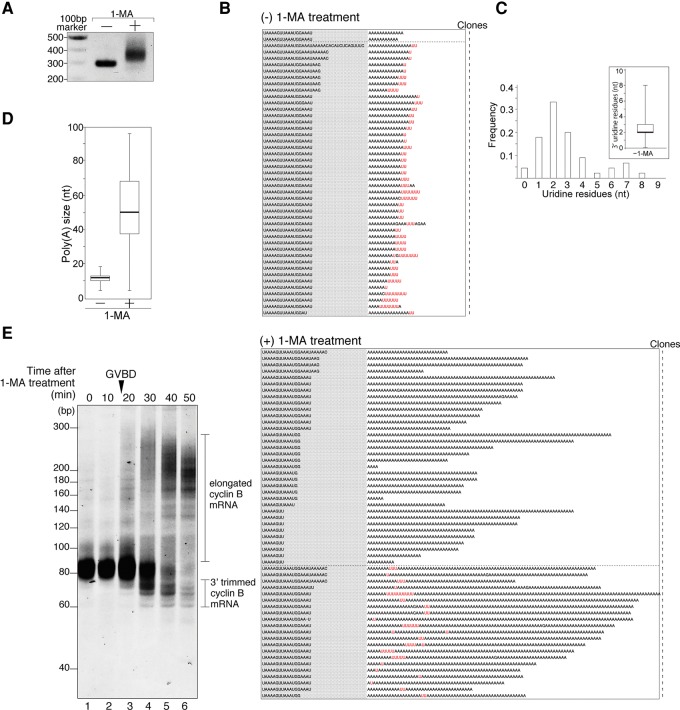
FIGURE 1.
Uridylated short poly(A) tail of cyclin B mRNA in starfish oocytes and nonuridylated long poly(A) tail of cyclin B mRNA in oocytes stimulated with the hormone 1-MA. (A) Elongation of the 3′ region of mRNA after hormonal stimulation. Adaptor-ligated cyclin B mRNAs from oocytes treated with (+) or without (−) 1-MA were RT-PCR amplified. The products were separated by agarose gel electrophoresis and visualized by ethidium bromide staining. (B) Sequencing results of the 3′ terminal region of cyclin B mRNA from oocytes treated with [(+) 1-MA treatment] or without 1-MA [(−) 1-MA treatment]. Gray shaded sequences show the 3′ terminal portion of the 3′ UTR, and the following sequences are the tail regions of cyclin B mRNA. The number of clones obtained is indicated. (C) Histogram of the number of uridine residues at the 3′ ends of cyclin B mRNAs in starfish oocytes not subjected to hormonal stimulation. Inset shows box-plot analysis of oligo(U) tail length. The 25th and 75th percentiles are shown by box edges, the median value is indicated by the thick line, and whiskers show the maximum and minimum values. (D) Box plot analysis of poly(A) tail length of cyclin B mRNAs in oocytes. Median of poly(A) size increased from 12 to 50 nt after hormonal stimulation. (E) Trimming activity at the 3′ region of cyclin B mRNA after hormonal stimulation. GVBD occurred 18 min after 1-MA treatment. At the indicated time after hormonal stimulation, total RNA was purified from oocytes, and adaptor-ligated cyclin B mRNAs were RT-PCR amplified using 3′ adaptor Rev primer 1 and sfcycB short Fwd primer. The PCR products were subjected to high-resolution acrylamide gel electrophoresis (15%) and visualized by SYBR-Green I staining.