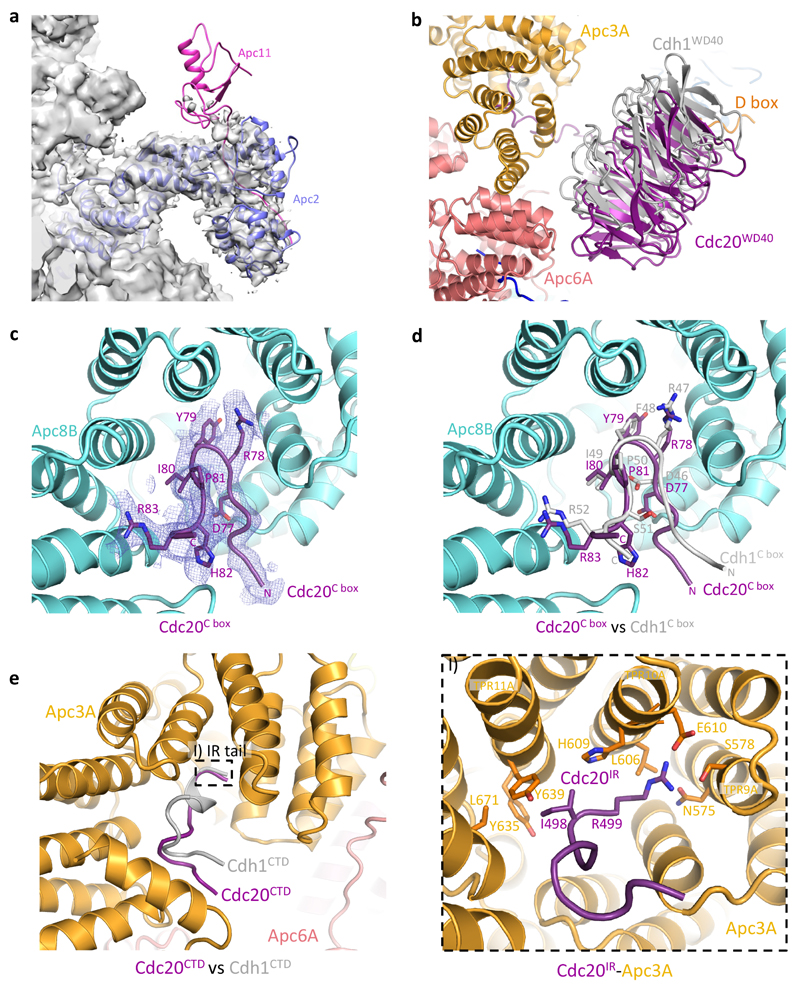
Extended Data Figure 3. Comparison of Cdc20 and Cdh1 association to the APC/C.
a, The catalytic module (Apc2-Apc11) of the APC/CCdc20-Hsl1 complex is flexible and almost no density accounting for Apc11 (pink, modelled based on the structure of APC/CCdh1-Emi1, PDB 4UI9)23 could be observed. b, The WD40 domain of Cdc20 (purple) occupies a similar position as Cdh1WD40 (grey), but it is displaced from the APC/C by as much as 10 Å. c, d, EM density for Cdc20C box allowed for ab initio model building and the C-box interaction with Apc8B (cyan) is well conserved between the two coactivators. e, Both Cdc20IR and Cdh1IR associates with Apc3A (orange), although the EM density for Cdc20IR is much weaker (not shown) and the C-terminal α-helix in Cdh1IR is absent.