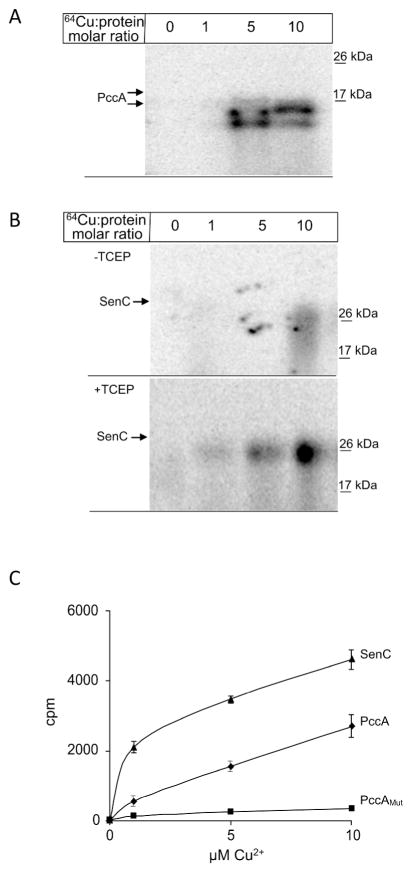
Figure 3. PccA is a copper binding protein.
(A) PccA was purified via a C-terminal Strep-tag and 10 μM of purified proteins were incubated with increasing concentrations of 64CuCl2. Samples were subsequently separated on native PAGE and analyzed by phosphor-imaging. (B) Purified SenC (10μM) was incubated with increasing concentrations of 64CuCl2 with and without prior reduction of the cysteine-containing copper binding motif by TCEP. Samples were then analyzed as in (A). (C) Quantification of the 64Cu binding to SenC (▲), or PccA (◆), or PccAMut, a mutant lacking the two conserved methionine residues of the Cu-binding motif of PCuAC chaperones (■). After 64Cu binding for 10 min at room temperature, samples were purified via Strep-tag and Cu binding was analyzed using a scintillation counter. The data displayed are the mean value of four independent experiments and the error bars represent the standard deviation.