Figure 9. A putative Cu delivery pathway for cbb3-Cox assembly in R. capsulatus.
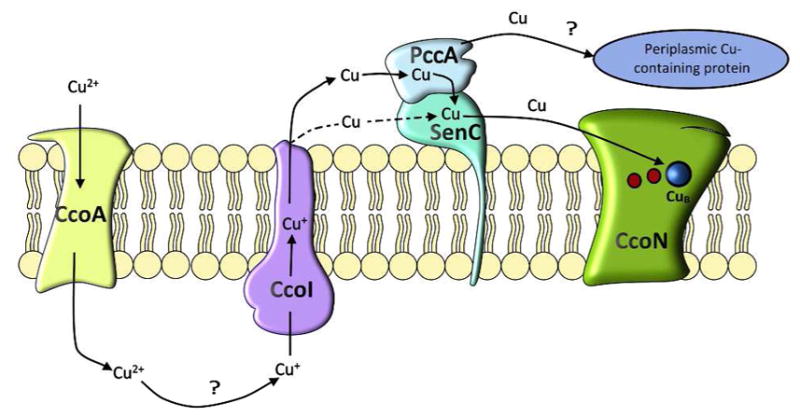
At low Cu concentrations, the MFS-type Cu transporter CcoA imports Cu2+, which is reduced to Cu+ in the cytoplasm by an unknown mechanism. The P1B-type ATPase CcoI transports Cu+ into the periplasm, where it is bound to PccA, which transfers it to the Sco1-homologue SenC and possibly to other periplasmic Cu-dependent proteins. SenC then delivers Cu+ to the CcoN subunit of cbb3-Cox (red dots indicate the two heme groups in CcoN). In the absence of PccA, Cu+ loading onto SenC and subsequent cbb3-Cox assembly is significantly reduced but still detectable (dashed arrows). At high Cu concentrations, cbb3-Cox assembly proceeds independently of CcoA, SenC and PccA, but still requires CcoI. Although SenC and PccA are dispensable for cbb3-Cox assembly at high Cu concentration, they are very important for regulating Cu homeostasis under these conditions. Absence of SenC leads to increased cytoplasmic Cu concentrations, which return to normal by the simultaneous elimination of PccA. A plausible explanation is the accumulation of Cu-loaded PccA in the absence of SenC, repressing Cu-export via CcoI and leading to increased cytoplasmic Cu pool.
