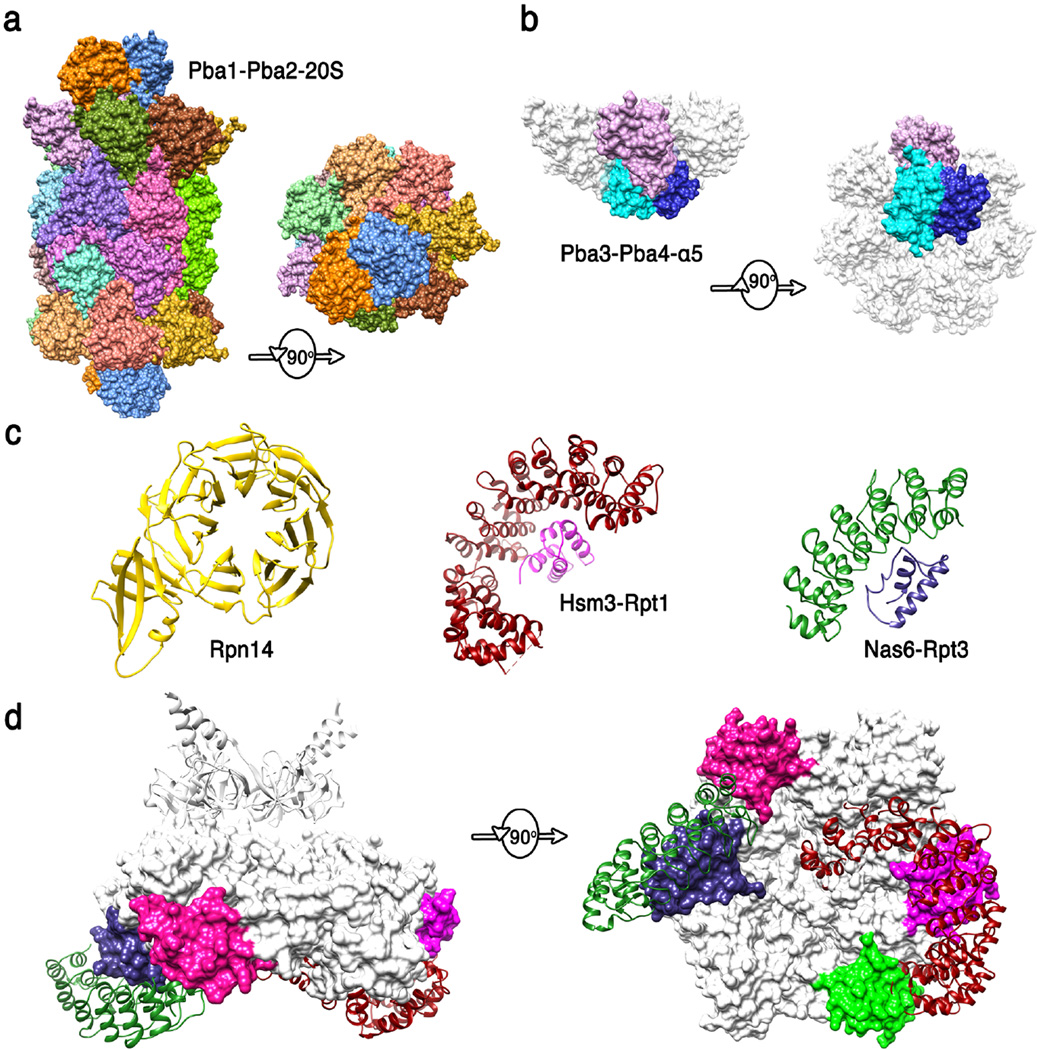
Figure 4. Structures of proteasome chaperones.
(a) Pba1-Pba2 (orange and blue) structure from (83) (pdb code 4g4s). Side and top views are shown of the complex with the 20S CP. The contacts seen in this structure are presumably maintained from the earliest stages of α-ring assembly to maturation of the 20S CP. (b) Pba3-Pba4 (shades of blue) structure from (100) (pdb code 2z5c). Side and bottom views are shown of this complex with α5, with the other α subunits modeled in white based on their structure in the mature 20S CP. This structure explains why Pba3-Pba4 are lost as β subunits are added to the assembling 20S CP. (c) Structures of 19S RP chaperones. Rpn14 (36) (pdb code 3acp), Hsm3 complex with C-terminal domain of Rpt1 (1) (pdb code 4a3v), and Nas6/gankyrin complex with the C-terminal domain of Rpt3 (54) (pdb code 2dzn). (d) Side and bottom model of Hsm3 and Nas6 docked onto the Rpt hexamer model. Substantial steric clashes would occur with the 20S CP (not shown) in the 26S proteasome, and minor steric clashes are suggested with Rpt subunits, consistent with the mechanisms that the 19S RP chaperones modulate interactions between ATPase subcomplexes and with the 20S CP. The C-terminal domains of Rpt5 and Rpt6 that bind Nas2 and Rpn14 are colored green and pink, respectively.