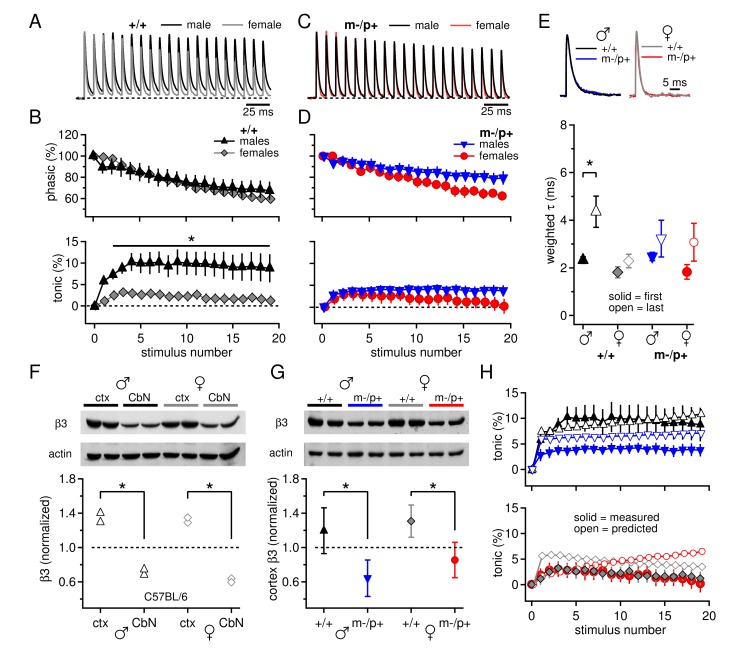
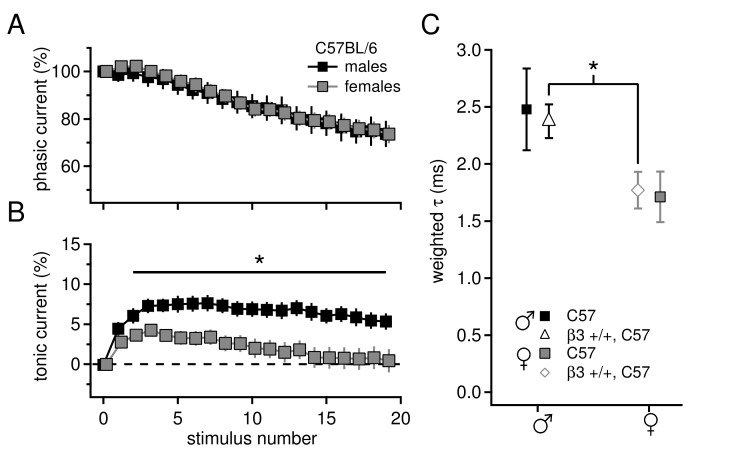
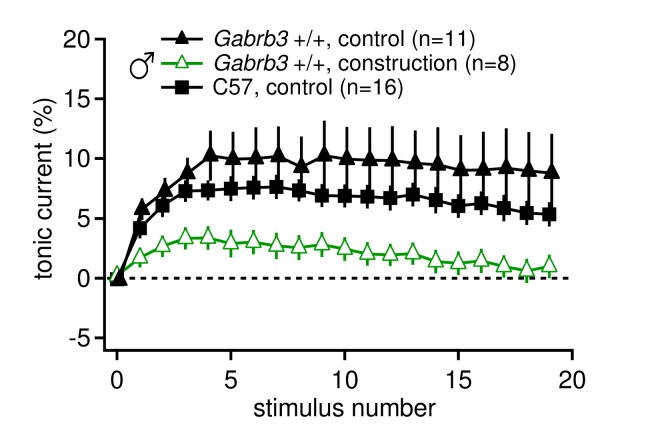
Figure 1. Sex differences in CbN synaptic currents and sex-specific responses to the Gabrb3 m-/p+ mutation.
(A) 100-Hz trains of synaptic currents evoked in CbN cells from male and female wild-type mice, normalized to the first peak. Dotted line, baseline holding current. (B) Mean amplitudes of phasic (upper panel) and tonic (lower panel) synaptic currents as a percentage of the first peak synaptic current vs. stimulus number. Dotted line, 0 current. (C,D) As in A, B, but for cells from male and female m-/p+ mice. (E) Top: example IPSCs evoked by a single stimulus, normalized to the peak current. Bottom: Solid symbols: weighted τdecay for IPSCs from a single stimulus. Open symbols: weighted τdecay for the last IPSC in the train. (F) Representative blot (top) and quantification (bottom) for β3 subunit expression in the cerebellar cortex vs. the cerebellar nuclei in C57BL/6 mice. Each symbol represents the normalized value for one lane. (G) Representative blot (top) and quantification (bottom) for normalized β3 subunit protein expression in the cerebellar cortex of Gabrb3 mice. (H) Solid symbols: measured tonic current, re-plotted from (B) and (D). Open symbols: predicted tonic current, calculated from the weighted τdecay of the first and last IPSCs. Symbol color code as in (E). In all figures, data are plotted as mean ± SEM. Asterisks indicates statistically significant differences.