Abstract
Laser-evoked potentials (LEPs) are brain responses to laser radiant heat pulses and reflect the activation of Aδ nociceptors. LEPs are to date the reference standard technique for studying nociceptive pathway function in patients with neuropathic pain. To find out whether LEPs also provide a useful neurophysiological tool for assessing antinociceptive drug efficacy, in this double-blind placebo-controlled study we measured changes induced by the analgesic tramadol on LEPs in 12 healthy subjects. We found that tramadol decreased the amplitude of LEPs, whereas placebo left LEPs unchanged. The opioid antagonist naloxone partially reversed the tramadol-induced LEP amplitude decrease. We conclude that LEPs may be reliably used in clinical practice and research for assessing the efficacy of antinociceptive drugs.
Keywords: Laser-evoked potentials, Tramadol, Opioid, Insula, Cingulate cortex
1. Introduction
Laser-generated radiant heat pulses selectively activate Aδ and C nociceptors, and evoke ‘late’ brain potentials (LEPs) that depend on the activation of Aδ fibres (Treede, 2003). LEPs consist of a lateralized component (N1-LEP) probably generated in the secondary somatosensory area (SII) and in the insular cortex (IC), and a vertex potential consisting of an N2–P2 complex; the N2-LEP component is believed to reflect neuronal activity in IC networks and possibly the anterior cingulate cortex (ACC) whereas the P2-LEP originates from the ACC alone (Garcia-Larrea et al., 2003). According to recent European guidelines, LEPs are considered the most reliable neurophysiological tool for assessing nociceptive pathway function in patients with neuropathic pain. However, LEPs have been used sparely as a tool for assessing analgesic treatments (Cruccu et al., 2004). Accordingly, few reports have described opioid-related changes of LEPs (Petersen-Felix et al., 1996; Lorenz et al., 1997). In addition, it is unknown whether an acute dose of an analgesic drug alters LEP components.
To provide evidence on the usefulness of LEPs as a tool for assessing the efficacy of antinociceptive drugs, in this study we investigated whether the three LEP components (N1, N2, and P2) were modulated by a single therapeutic dose of tramadol in healthy subjects. Tramadol analgesia results from a monoaminergic effect by tramadol itself and an opioid effect of its metabolite (+)-M1 (Desmeules et al., 1996; Enggaard et al., 2006; Berrocoso et al., 2007). We chose tramadol because it is probably the most widely used central-acting analgesic drug and randomized controlled trials have shown that it is an effective treatment for neuropathic pain (Attal et al., 2006; Hollingshead et al., 2006).
2. Methods
Twelve healthy volunteers aged 25–32 years participated in the experiments. All procedures, approved by the local Ethical Committee, were in accordance with the Helsinki Declaration and IASP’s guidelines for pain research in humans. All subjects gave their informed consent.
2.1. Laser stimulation
We used a Nd:YAP laser stimulator with fibre–optic guidance (Electronic Engineering, Florence, Italy). Laser stimuli were set to induce a clear painful pin-prick (intensity 119.4–150 mJ/mm2; duration 5 ms; diameter ~4 mm) and delivered to the right hand dorsum. The laser beam was slightly moved after each stimulus and the interstimulus interval was varied pseudorandomly (10–15 s). To keep the attention stable subjects were asked to mentally count the number of stimuli.
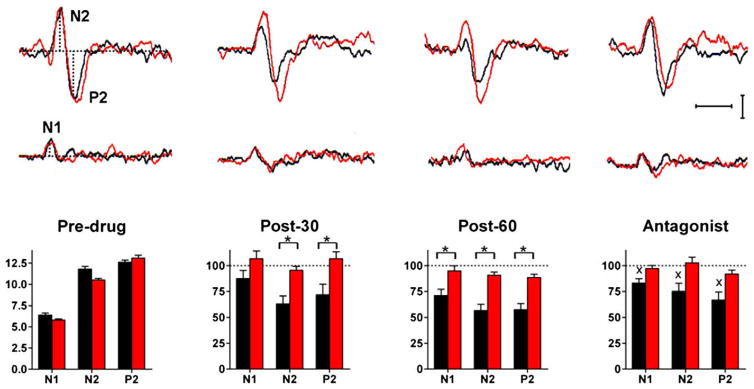
Subjects, wearing protective goggles, rested comfortably on a couch, keeping their eyes open. Aδ-LEP components were recorded through disk electrodes from the scalp: N1 from T3, T4 referenced to Fz and N2–P2 from Cz referenced to the nose. Electroculographic recordings monitored possible eye movements or blinks. For each session, perceptive threshold was assessed, and two series of 15 trials devoid of artefacts were averaged off line and stored on disk. We measured the peak latencies of the lateralized N1 and the vertex N2 and P2 components. The amplitude of all components (N1, N2, and P2) was measured from baseline to peak (Fig. 1).
Fig. 1.
Upper panel: laser-evoked potentials (LEPs) recordings during tramadol (black) and placebo (red) sessions in a representative subject. Each trace is the mean of 30 trials. Horizontal calibration: 200 ms; vertical calibration: 5 μV. Lower panel: Mean pre-drug values and post-drug changes in amplitude of the N1, N2, and P2 components of LEPs during tramadol (black) and placebo (red) sessions. Post-drug values are normalized to pre-drug values.*: significant difference between tramadol and placebo. X: significant difference between the 60-min and antagonist blocks. Whereas placebo was ineffective, tramadol reduced and naloxone partially recovered all LEP components.
2.2. Experimental procedure
We conducted a double-blind crossover study. All subjects underwent two separate sessions, one with tramadol and the other with placebo. The order of the two sessions was randomly alternated among subjects. The LEP measures were taken off-line by two of the investigators, who were unaware of the type of session (drug or placebo). Each session consisted of 4 recording blocks: (1) before i.m. injection of tramadol 100 mg/2 ml or saline 2 ml (predrug); (2) 30 min after drug or placebo (post-30); (3) 60 min after drug or placebo (post-60); and (4) an “antagonist block” immediately after i.v. injection of naloxone 0.4 mg/1 ml (in the tramadol session) or saline 1 ml (in the placebo session).
2.3. Statistical analysis
Because perceptive threshold and LEP data had a Gaussian distribution (Kolmogorov–Smirnov test) they were analyzed with parametric methods. Differences between blocks (pre-drug, 30-min, and 60-min) and between drug and placebo sessions were analyzed with two-way analysis of variance (ANOVA) for repeated measures. Changes between the last post-drug block and the antagonist block were analyzed with Student’s t test for paired data. To compare differences between LEP components (N1, N2, and P2) we also calculated regression lines and their slopes and evaluated their differences with one-way ANOVA for repeated measures and Bonferroni post-hoc test.
3. Results
Pre-drug values were similar in the placebo and tramadol sessions (Table 1). Placebo left the perceptive threshold in the healthy subjects unaffected while tramadol slightly increased it (from 69.8 ± 4.5 mJ/mm2 in the pre-drug block to 78.3 ± 3.8 mJ/mm2 in the 60-min block). These tramadol-induced changes did not differ from placebo (p > 0.10). No subject showed or reported sedation.
Table 1.
Comparison of pre-drug values.
| Tramadol session | Placebo session | |
|---|---|---|
| Perceptive threshold (mJ/mm2) | 69.8 ± 4.5 | 67.8 ± 4.8 |
| N1 latency (ms) | 185 ± 5.3 | 181 ± 4.5 |
| N1 amplitude (μV) | 6.4 ± 0.8 | 5.8 ± 0.5 |
| N2 latency (ms) | 207 ± 4.3 | 204 ± 3.3 |
| N2 amplitude (μV) | 11.8 ± 1,1 | 10.5 ± 0.7 |
| P2 latency (ms) | 278 ± 7.9 | 283 ± 8.0 |
| P2 amplitude (μV) | 12.6 ± 0.9 | 13.1 ± 1.1 |
No pre-drug value was significantly different between the tramadol and placebo sessions.
Placebo injection left all LEP components unchanged (p > 0.10). Although tradamol also left the three LEP components unchanged in latency (p > 0.10), two-way ANOVA showed that this drug significantly reduced their amplitude over time (Df 2; N1: F = 7.605, p < 0.01; N2: F = 26.27, p < 0.0001; and P2: F = 13.28, p < 0.0001), and that the tramadol-induced changes all differed significantly from placebo (Df 1; N1: F = 6.7, p = 0.01; N2: F = 24.03, p < 0.0001; and P2: F = 17.16, p = 0.0004).
Naloxone administration partially recovered the amplitude of all LEP components (from values at the 60-min block: N1: p = 0.04; N2: p = 0.03; and P2: p = 0.04), which remained about 30% smaller than at baseline. Saline left unchanged all components (N1: p = 0.4; N2: p = 0.2; and P2: p = 0.4).
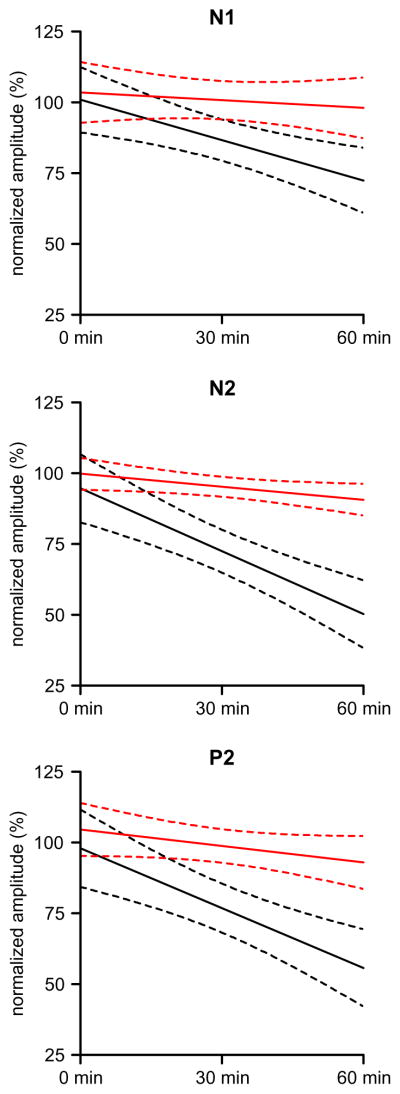
To investigate possible differences among the N1, N2, and P2 LEP components, we calculated the regression lines for the overall tramadol-induced effect throughout the experimental session for each subject, and then compared the mean slopes (Fig. 2). In the placebo session, although the mean regression lines shown in Fig. 2 (red lines) seem to decay with time, the three slopes did not differ significantly from zero (p > 0.10). In the tramadol session, the regressions were always significant and the slopes for all three LEP components differed significantly from zero (N1: p = 0.001; N2: p < 0.0001; and P2: p < 0.0001), but the N2 and P2 slopes were steeper than that of N1. One-way ANOVA of the slope values for N1, N2, and P2 was highly significant (p < 0.001), with significant differences between N1 and the other two components (p < 0.01), though not between N2 and P2. A calculation of the regression lines projected to 120 min resulted in a 45% reduction for N1 and 95% for N2.
Fig. 2.
Regression lines for tramadol (black) and placebo (red) sessions. In the placebo session the slope was never significantly different from zero. In the tramadol session the slope was significantly different from zero for all the three LEP components, but the N2 and P2 slopes were steeper than that of N1 (p < 0.001).
4. Discussion
Our experiments in healthy subjects demonstrate that a single dose of the analgesic drug tramadol attenuates LEPs acting predominantly on the N2 and P2 components. The ability of naloxone to partly reverse the effects of tramadol suggest that tramadol altered LEPs mainly through an action on opioid receptors.
Several studies performed in rodents have shown that the analgesic effect of tramadol is mediated by both opioidergic and monoaminergic actions (specifically through inhibition of norepinephrine and serotonin reuptake) (Rojas-Corrales et al., 2005; Berrocoso et al., 2007). In humans, the importance of the two actions seems to depend much on the individual ability to metabolise tramadol and thus produce its opioidergic metabolite (+)-M1 through O-demethylation by CYP2D6 (Desmeules et al., 1996; Enggaard et al., 2006).
The experiments reported here suggest that the changes induced by tramadol on LEPs were predominantly due to its opioidergic action. According to the available data in humans, the opioidergic metabolite (+)-M1 is present and active 15–90 min (i.e. when our measures were taken) after tramadol administration in extensive metabolisers, i.e. in 93% of the Caucasian population (Desmeules et al., 1996; Poulsen et al., 1996; Enggaard et al., 2006). In keeping with an opioidergic mechanism for the action of tramadol, we found that naloxone did restore significantly the LEP amplitude. The naloxone action was however incomplete, particularly on the N2- and P2-LEP components, which remained about 30% smaller than pre-drug values.
In the placebo session, the LEP components showed (Fig. 2) a slight attenuation over time (though non significant), as it may be expected since these evoked potentials undergo some degree of habituation with repeated blocks of stimulations (Valeriani et al., 2003). The difference between placebo and tramadol, however, was always highly significant.
Whereas tramadol suppressed LEPs by about 50%, it only slightly increased the perceptive threshold. In experimental pain models in general, the opioid effects on pain threshold are much less pronounced than the effects on pain tolerance (Angst et al., 2001; Skarke et al., 2003), but similar findings have also been reported for monoaminergic drugs (Poulsen et al., 1995). Furthermore the perceptive threshold to laser stimuli is in our experience less sensitive than LEP amplitude to drug effects probably because even a minimal afferent input is sufficient to preserve it normal (Truini and Cruccu, 2008).
Tramadol acted differentially on the three LEP components. It attenuated N1, a LEP component probably generated by SII and insular cortices, by about 25%, and N2 and P2, generated by insular and cingulate cortices, by about 50%. This differential modulation suggests that through its opioidergic effect, tramadol, acting at spinal and supraspinal levels, may differentially affect cortical regions. Hence the three LEP components probably undergo the same drug effect at dorsal horn level whereas the drug action differs between SII, IC and ACC. This hypothesis agrees with the notion of a differential potency of opioid analgesia on different brain regions (Scharein and Bromm, 1995) and recent neuroimaging studies reporting that the opioid-receptor density is stronger in the ACC than the somatosensory cortices (Baumgärtner et al., 2006; Kupers, 2006).
Finally, in our study tramadol modulated N2- and P2-LEPs in a similar manner, thus suggesting that the opioid-receptor density is similar in the insular and cingulate cortices. This finding parallels our in vitro rat experiments (Sudbury and Avoli, 2007; Panuccio et al., 2009) and neuroimaging studies in humans (Baumgärtner et al., 2006).
Acknowledgments
This study was supported by grants from La Sapienza University of Rome, Italy, the Canadian Institutes of Health Research (Grant MT-8109), and the Savoy Foundation, Canada. The authors have no conflict of interest to declare.
References
- Angst MS, Drover DR, Lötsch J, Ramaswamy B, Naidu S, Wada DR, et al. Pharmacodynamics of orally administered sustained-release hydromorphone in humans. Anesthesiology. 2001;94:63–73. doi: 10.1097/00000542-200101000-00014. [DOI] [PubMed] [Google Scholar]
- Attal N, Cruccu G, Haanpaa M, Hansson P, Jensen TS, Nurmikko T, et al. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006;13:1153–69. doi: 10.1111/j.1468-1331.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- Baumgärtner U, Buchholz HG, Bellosevich A, Magerl W, Siessmeier T, Rolke R, et al. High opiate receptor binding potential in the human lateral pain system. Neuroimage. 2006;30:692–9. doi: 10.1016/j.neuroimage.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Berrocoso E, De Benito MD, Micò JA. Role of serotonin 5-HT1A and opioid receptors in the antiallodynic effect of tramadol in the chronic constriction injury model of neuropathic pain in rats. Psychopharmacology (Berl) 2007;193:97–105. doi: 10.1007/s00213-007-0761-8. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Anand P, Attal N, Garcia-Larrea L, Haanpaa M, Jorum E, et al. EFNS guidelines on neuropathic pain assessment. Eur J Neurol. 2004;11:153–62. doi: 10.1111/j.1468-1331.2004.00791.x. [DOI] [PubMed] [Google Scholar]
- Desmeules JA, Piguet V, Collart L, Dayer P. Contribution of monoaminergic modulation to the analgesic effect of tramadol. Br J Clin Pharmacol. 1996;41:7–12. doi: 10.1111/j.1365-2125.1996.tb00152.x. [DOI] [PubMed] [Google Scholar]
- Enggaard TP, Poulsen L, Arendt-Nielsen L, Brøsen K, Ossig J, Sindrup SH. The analgesic effect of tramadol after intravenous injection in healthy volunteers in relation to CYP2D6. Anesth Analg. 2006;102:146–50. doi: 10.1213/01.ane.0000189613.61910.32. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Frot M, Valeriani M. Brain generators of laser-evoked potentials: from dipoles to functoonal significance. Neurophysiol Clin. 2003;33:279–92. doi: 10.1016/j.neucli.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Hollingshead J, Duhmke RM, Cornblath DR. Tramadol for neuropathic pain. Cochrane Database Syst Rev. 2006;3:CD003726. doi: 10.1002/14651858.CD003726.pub3. [DOI] [PubMed] [Google Scholar]
- Kupers R. Brain imaging of pain. In: Cervero F, Jensen T, editors. Pain Handbook of clinical neurology. Vol. 81. Amsterdam: Elsevier; 2006. pp. 481–97. [DOI] [PubMed] [Google Scholar]
- Lorenz J, Beck H, Bromm B. Differential changes of laser evoked potentials, late auditory evoked potentials and P300 under morphine in chronic pain patients. Electroencephalogr Clin Neurophysiol. 1997;104:514–21. doi: 10.1016/s0168-5597(97)00064-6. [DOI] [PubMed] [Google Scholar]
- Panuccio G, Curia G, Colosimo A, Cruccu G, Avoli M. Epileptiform synchronization in the cingulate cortex. Epilepsia. 2009;50:521–36. doi: 10.1111/j.1528-1167.2008.01779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen-Felix S, Arendt-Nielsen L, Bak P, Fischer M, Zbinden AM. Psychophysical and electrophysiological responses to experimental pain may be influenced by sedation: comparison of the effects of a hypnotic (propofol) and an analgesic (alfentanil) Br J Anaesth. 1996;77:165–71. doi: 10.1093/bja/77.2.165. [DOI] [PubMed] [Google Scholar]
- Poulsen L, Arendt-Nielsen L, Brosen K, Nielsen KK, Gram LF, Sindrup SH. The hypoalgesic effect of imipramine in different human experimental pain models. Pain. 1995;60:287–93. doi: 10.1016/0304-3959(94)00142-2. [DOI] [PubMed] [Google Scholar]
- Poulsen L, Arendt-Nielsen L, Brøsen K, Sindrup SH. The hypoalgesic effect of tramadol in relation to CYP2D6. Clin Pharmacol Ther. 1996;60:636–44. doi: 10.1016/S0009-9236(96)90211-8. [DOI] [PubMed] [Google Scholar]
- Rojas-Corrales MO, Berrocoso E, Micó JA. Role of 5-HT1A and 5-HT1B receptors in the antinociceptive effect of tramadol. Eur J Pharmacol. 2005;511:21–6. doi: 10.1016/j.ejphar.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Scharein E, Bromm B. Comparative evaluation of analgesic efficacy of drugs. In: Bromm B, Desmedt JE, editors. Pain and the brain – from nociception to cognition. Advances in pain research and therapy. Vol. 22. New York: Raven Press; 1995. p. 7. [Google Scholar]
- Skarke C, Darimont J, Schmidt H, Geisslinger G, Lötsch J. Analgesic effects of morphine and morphine-6-glucuronide in a transcutaneous electrical pain model in healthy volunteers. Clin Pharmacol Ther. 2003;73:107–21. doi: 10.1067/mcp.2003.5. [DOI] [PubMed] [Google Scholar]
- Sudbury JR, Avoli M. Epileptiform synchronization in the rat insular and perirhinal cortices in vitro. Eur J Neurosci. 2007;26:3571–82. doi: 10.1111/j.1460-9568.2007.05962.x. [DOI] [PubMed] [Google Scholar]
- Treede R-D. Neurophysiological studies of pain pathways in peripheral and central nervous system disorders. J Neurol. 2003;250:1152–61. doi: 10.1007/s00415-003-0237-7. [DOI] [PubMed] [Google Scholar]
- Truini A, Cruccu G. Laser evoked potentials in patients with trigeminal disease: the absence of A-delta potentials does not unmask C-fibre potentials. Clin Neurophysiol. 2008;119:1905–8. doi: 10.1016/j.clinph.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Valeriani M, de Tommaso M, Restuccia D, Le Pera D, Guido M, Iannetti GD, et al. Reduced habituation to experimental pain in migraine patients: a CO2 laser evoked potential study. Pain. 2003;105:57–64. doi: 10.1016/s0304-3959(03)00137-4. [DOI] [PubMed] [Google Scholar]