Abstract
Mesial temporal lobe epilepsy (MTLE) is characterized in humans and in animal models by a seizure-free latent phase that follows an initial brain insult; this period is presumably associated to plastic changes in temporal lobe excitability and connectivity. Here, we analyzed the occurrence of interictal spikes and high frequency oscillations (HFOs; ripples: 80–200 Hz and fast ripples: 250–500 Hz) from 48 h before to 96 h after the first seizure in the rat pilocarpine model of MTLE. Interictal spikes recorded with depth EEG electrodes from the hippocampus CA3 area and entorhinal cortex (EC) were classified as type 1 (characterized by a spike followed by a wave) or type 2 (characterized by a spike with no wave). We found that: (i) there was a switch in the distribution of both types of interictal spikes before and after the occurrence of the first seizure; during the latent phase both types of interictal spikes predominated in the EC whereas during the chronic phase both types of spikes predominated in CA3; (ii) type 2 spike duration decreased in both regions from the latent to the chronic phase; (iii) type 2 spikes associated to fast ripples occurred at higher rates in EC compared to CA3 during the latent phase while they occurred at similar rates in both regions in the chronic phase; and (iv) rates of fast ripples outside of spikes were higher in EC compared to CA3 during the latent phase. Our findings demonstrate that the transition from the latent to the chronic phase is paralleled by dynamic changes in interictal spike and HFO expression in EC and CA3. We propose that these changes may represent biomarkers of epileptogenicity in MTLE.
Keywords: Temporal lobe epilepsy, Epileptogenesis, High-frequency oscillations, Interictal spikes
Introduction
Mesial temporal lobe epilepsy (MTLE), one of the most common forms of partial epilepsy, is characterized by recurrent seizures that originate from the hippocampus, amygdala or entorhinal cortex (EC), and that often occur after a latent phase of many years following an initial brain insult such as febrile seizures, encephalitis, or status epilepticus (Engel, 1996; Gloor, 1997; Salanova et al., 1994). This latent phase is presumably characterized by reorganization of neural networks and by changes in cellular excitability (Dudek and Staley, 2012). Interictal spikes, and more recently high-frequency oscillations (HFOs, 80–500 Hz), recorded from patients with MTLE and in animal models mimicking this disorder have been considered markers of abnormal neural network activity (Jefferys et al., 2012a,b). However, the relation between interictal spikes, HFOs and epileptogenesis remains unclear.
A few years ago, we found that interictal spikes recorded from the hippocampus CA3 area, EC, and amygdala in pilocarpine-treated epileptic rats change in duration and rate of occurrence following the first seizure (Bortel et al., 2010). More recently, Chauvière et al. (2012) have reported in the hippocampus CA1 area the occurrence of two types of interictal discharges following pilocarpine- or kainic acid-induced status epilepticus: type 1 consisted of a spike followed by a wave whereas type 2 was characterized by a spike without wave. They also found changes over time in the rate of occurrence of these two types of interictal discharge; the rate of occurrence of type 1 spikes decreased from the latent to the chronic phase whereas the rate of occurrence of type 2 spikes increased. Therefore, both studies suggested that interictal spikes may represent a biomarker of epileptogenicity (i.e., the electrophysiological signature of the changes in brain excitability leading to the chronic epileptic condition).
To date, no study has addressed the relation between interictal spikes and HFOs during epileptogenesis. HFOs, categorized as ripples (80–200 Hz) and fast ripples (250–500 Hz), occur in the EEG of epileptic patients and animals in coincidence with interictal spikes but also in their absence, and they are thought to reflect the activity of dysfunctional neural networks (Jacobs et al., 2012; Jefferys et al., 2012a,b; Zijlmans et al., 2009). In addition, both clinical and experimental studies support the view that HFOs are better markers than interictal spikes to identify seizure onset zones (Jacobs et al., 2008, 2012; Jefferys et al., 2012a,b; Jiruska et al., 2010a,b; Urrestarazu et al., 2007). Finally, it has been reported in pilocarpine-treated animals that during the chronic phase there is a high correlation between interictal spikes associated with HFOs and seizure onset zones (Lévesque et al., 2011). Therefore, in this study, we used the pilocarpine model of MTLE to analyze the evolution of interictal spikes and the occurrence of HFOs at the transition from the latent to the chronic phase. We recorded the hippocampus CA3 area and EC, as they are in most cases the seizure onset zones in this model of MTLE (Bortel et al., 2010; Lévesque et al., 2011, 2012).
Materials and methods
Pilocarpine treatment
Male Sprague–Dawley rats (250–300 g; n = 9) were acquired from Charles River Laboratories (St-Constant, Qc, Canada) and let habituate to the environment for 72 h before pilocarpine treatment. On the day of injection, they were administered scopolamine methylnitrate (1 mg/kg i.p.; Sigma-Aldrich, Canada) and 30 min later a single dose of pilocarpine hydrochloride (380 mg/kg, i.p.; Sigma-Aldrich, Canada) (Bortel et al., 2010; Lévesque et al., 2011, 2012). Their behavior was scored according to the Racine scale (Racine, 1972), and status epilepticus was defined as continuous stage 5 seizures. Status epilepticus was terminated after 1 h by injection of diazepam (5 mg/kg, s.c.; CDMV, Canada) and ketamine (50 mg/kg, s.c.; CDMV, Canada) (Martin and Kapur, 2008).
Implantation of bipolar depth electrodes
Two days after status epilepticus, rats were anesthetized with isoflurane (3%) in 100% O2. Bipolar electrodes (20–30 kΩ; 5–10 mm in length; distance between exposed tips: 500 μm) were implanted in the CA3 area of the ventral hippocampus (AP: −4.3, ML: ±4, DV: −7.8) and EC (AP: −8.6, ML: ±5.2, DV: −6.8). Screws placed in the frontal bone were used as reference and ground (cf., Bortel et al., 2010; Lévesque et al., 2011). All procedures were approved by the Canadian Council of Animal Care and all efforts were made to minimize the number of animals used and their suffering.
Local field potential recordings
After surgery, rats were housed individually in custom-made Plexiglas boxes (30 × 30 × 40 cm) and let habituate to the environment for 24 h. Electrodes were then connected to a multichannel cable and electrical swivel (Slip ring T13EEG, Air Precision, France; or Commutator SL 18C, HRS Scientific, Canada) and EEG-video monitoring (24 h per day) was performed. EEGs were amplified via an interface kit (Mobile 36ch LTM ProAmp, Stellate, Montreal, QC, Canada), low-pass filtered at 500 Hz and sampled at 2 kHz per channel. Infrared cameras were used to record day/night video files that were time-stamped for integration with the electrophysiological data using monitoring software (Harmonie, Stellate). Throughout the recordings, animals were placed under controlled conditions (22 ± 2 °C, 12 h light/dark schedule) and provided with food and water ad libitum. EEG-video recordings were performed up to 15 days after status epilepticus.
Selection of epochs
Spontaneous seizures started 6.1 (±0.7) days after status epilepticus. EEG recordings were extracted at 5 time points before the occurrence of the first seizure (defined as time 0): −48 h, −36 h, −24 h, −12 h, and −2 h. Six time points after the first seizure were also selected for analysis: +2 h, +12 h, +24 h, +48 h, +72 h, and +96 h. For each time point, 10 min epochs were selected in each rat. Only epochs of slow-wave sleep were used for analysis, because of the low rates of movement artifacts and since HFOs are more prominent during this sleep state (Bagshaw et al., 2009; Staba et al., 2004). Slow-wave sleep epochs were selected based on the occurrence of EEG activity in the 1–6 Hz range. During these periods, rats were immobile and in a curled body position. In order to minimize the effects of seizure occurrence during the chronic phase, the 10 min epochs were selected from periods at least 1 h before or after seizures. Overall, epochs of slow-wave sleep were extracted in a range of ±2 h from the time point (e.g., for the epoch at −48 h, it was selected from −46 to −50 h before first seizure occurrence). EEGs were then exported to Matlab 8.1 (R2013a) (Mathworks, Natick, MA, USA) and analyzed off-line using custom-built routines. The original epochs were reviewed and all sections with artifacts were removed from the analysis.
Classification and analysis of interictal spikes
Interictal spikes were detected based on threshold crossings (mean and standard deviation), calculated over the entire period for the 10 min epoch. The reviewer blind to the phase (latent or chronic) to which each epoch belonged, adapted the threshold visually to account for differences in detection performance (range: 2.7–3.5 standard deviations). Every event that crossed the threshold was then analyzed visually and only interictal spikes were kept for analysis. Interictal spikes were then classified visually into two types as in Chauvière et al. (2012). Type 1 spikes were characterized by a spike followed by a wave that was clearly distinguishable from the background, whereas type 2 spikes were characterized by a spike without a wave (see Figs. 1, 2 and 3). The duration of the spike component was calculated from the first deflection from baseline to the return to baseline. The duration of the wave in type 1 spikes was calculated from the end of the spike component to the return to baseline after the wave.
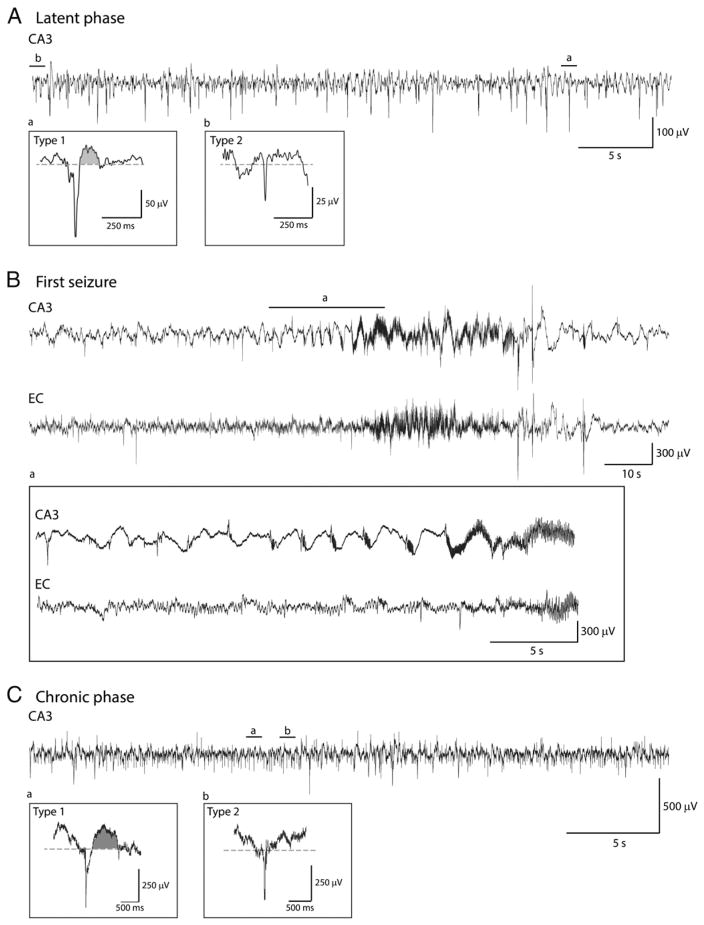
Fig. 1.
Representative examples of type 1 and type 2 interictal spikes recorded from the CA3 region in a pilocarpine-treated rat. A. EEG recordings obtained during the latent phase showing type 1 (a) and type 2 (b) spikes. B. First spontaneous seizure recorded from this animal. Note the hypersynchronous-onset pattern (pre-ictal spikes at a frequency of 0.4–0.7 Hz) that occurs in CA3, which also represents the seizure onset area. C. EEG recordings obtained during the chronic phase showing type 1 (a) and type 2 (b) spikes. Shaded areas in Aa and Ca highlight the wave component of type 1 spikes. Abbreviations in this and the following figures are: CA3: hippocampus CA3 subfield; and EC: entorhinal cortex.
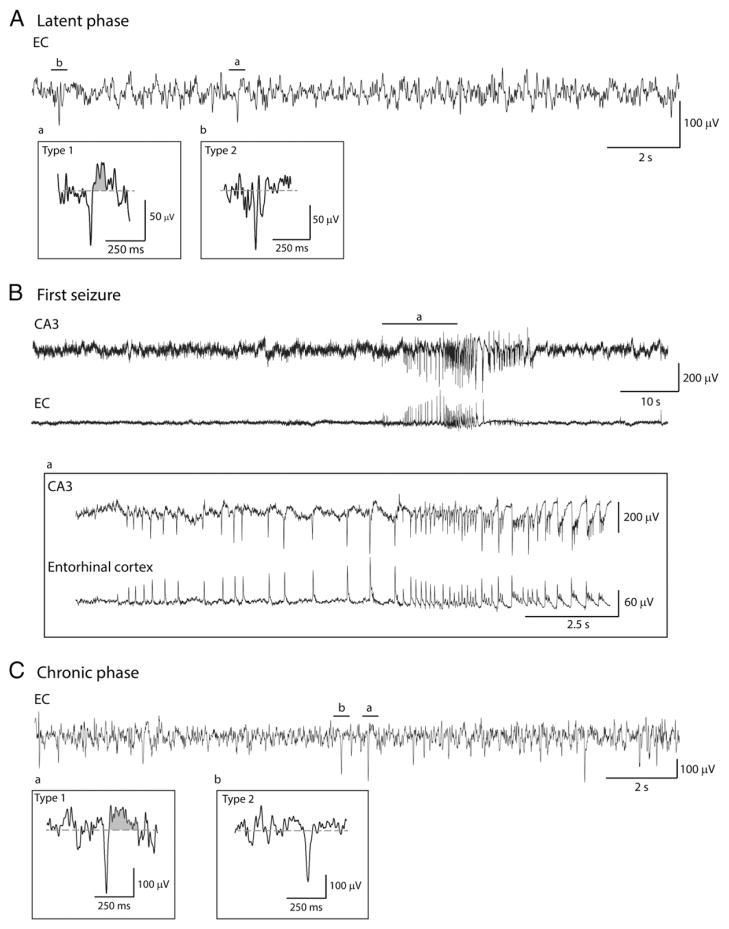
Fig. 2.
Representative examples of type 1 and type 2 interictal spikes recorded from the EC in a pilocarpine-treated rat. A. EEG recordings obtained during the latent phase showing type 1 (a) and type 2 (b) spikes. B. First spontaneous seizure recorded from this animal. Note also in this case that the seizure onset is characterized by a hypersynchronous pattern with pre-ictal spikes at a frequency of ranging from 1.6 to 2.3 Hz that occurs in both CA3 and EC but appears to start in the EC. C. EEG recordings obtained during the chronic phase showing type 1 (a) and type 2 (b) spikes.
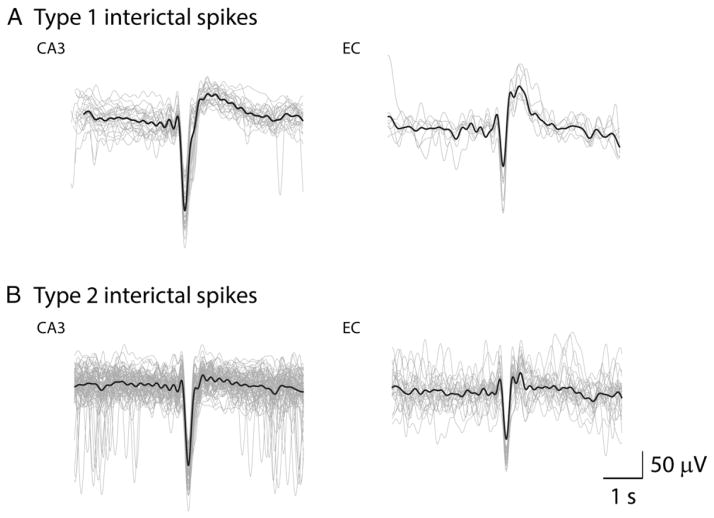
Fig. 3.
Superimposed spike waveform shapes for type 1 and type 2 interictal spikes in CA3 and EC. A. Type 1 spikes recorded in one rat from the CA3 and EC during the chronic period. B. Type 2 spikes recorded during the same time points in the CA3 and EC of the same animal. The solid lines represent the mean.
Analysis of high-frequency oscillations
The same epochs used for analyzing interictal spikes were used for HFO analysis. A multi-parametric algorithm was employed to identify oscillations in each frequency range (80–200 Hz and 250–500 Hz), using routines based on standardized functions (Matlab Signal Processing Toolbox). Raw EEG recordings were bandpass filtered in the 80–200 Hz and in the 250–500 Hz frequency range using a finite impulse response filter; zero-phase digital filtering was used to avoid phase distortion. Filtered EEGs from each region were then normalized using their own average RMS value calculated over the 10-min epoch. To be considered as an HFO candidate, oscillatory events in each frequency band had to show at least four consecutive cycles having amplitude of 3 SD above the mean. The time lag between two consecutive cycles had to be between 5 and 12.5 ms for ripples and between 2 and 4 ms for fast ripples (Lévesque et al., 2011, 2012; Salami et al., 2012). HFOs were considered as co-occurring with a spike if they occurred within a time window of ±500 ms from the peak of an interictal spike. Furthermore, special care was taken to avoid the detection of false HFOs: ripples were kept for analysis if they were only detected in the 80–200 Hz range, whereas fast ripples were kept if they were detected only in the 250–500 Hz range. Overlapping events, which could be caused by filtering spikes (Bénar et al., 2010) were thus excluded from the analysis. The ratio of interictal spikes occurring with HFOs to the total number of interictal spikes was calculated and changes over time were analyzed. The rates of occurrence of ripples and fast ripples on both types of spikes were calculated by dividing the number of ripples or fast ripples on spikes by the total number of the corresponding spike type. HFOs occurring outside of spikes were analyzed separately.
Statistical analysis
In order to study the evolution of rates of interictal spikes over time, values corresponding to the latent phase (48 h, 36 h, 24 h, 12 h and 2 h before the first seizure) were averaged together and were compared to values corresponding to the average value of the chronic phase time points (2 h, 12 h, 24 h, 48 h, 72 h and 96 h after the first seizure). Since values were not normally distributed, we used the non-parametric Kruskall–Wallis and Mann–Whitney–Wilcoxon tests. The level of significance was set at p < 0.05.
Results
Rates of occurrence of type 1 and type 2 spikes during the latent and chronic phases
We analyzed a total of 9328 spikes from CA3 and EC; 294 type 1 spikes and 4150 type 2 spikes were detected in CA3 while 288 type 1 spikes and 4596 type 2 spikes were detected in EC. Type 1 and type 2 spikes were seen during both latent and chronic phases. Examples of type 1 and type 2 spikes occurring in the CA3 area during the latent and chronic phases are shown in Figs. 1A and C, respectively, and the first seizure seen in this animal is illustrated in Fig. 1B. Examples of type 1 and type 2 spikes recorded from the EC during latent (panel A) and chronic (panel C) phases, along with the first spontaneous seizure (panel B), in another rat are illustrated in Fig. 2. The first spontaneous seizure recorded from these 9 pilocarpine-treated rats was characterized by CA3 (n = 3) or EC (n = 2) onset while in 4 animals no clear seizure onset zone could be defined (i.e., seizures appear to start at the same time in CA3 and EC). Seizure onset for 68 seizures recorded during the first 96 h of the chronic phase in these 9 animals was defined as follows: (i) CA3 in 18 seizures (27% of total); (ii) EC in 9 seizures (13% of total) and (iii) simultaneous onset in 41 seizures (60% of total). Consistent with the results reported by Toyoda et al. (2013), seizures started in the hippocampus in most of the recorded animals. Fig. 3 shows superimposed waveform shapes of type 1 and type 2 interictal spikes in CA3 and EC from a single animal and highlights the consistency in waveform shape across different interictal events.
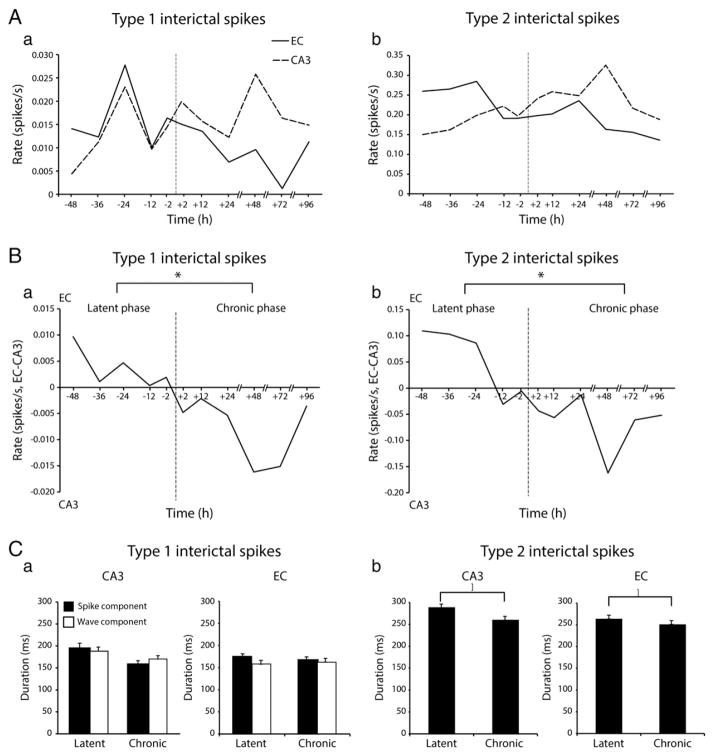
We then analyzed the rates of occurrence of type 1 and type 2 spikes. As illustrated in Fig. 4A, the rate of occurrence of type 1 (panel a) and type 2 (panel b) spikes was characterized in both CA3 and in EC by dynamic changes during the time period analyzed our experiments. To better compare these modifications, we subtracted the rate average for each time point in CA3 from the equivalent time-point in EC, and then we plotted the values over time (Fig. 4B). By doing so, we found that the occurrence of both type 1 and type 2 spikes was higher in EC compared to CA3 during the latent phase. This difference gradually decreased until the first spontaneous seizure occurred; this dynamic progression marked the start of the chronic phase, after which both types of interictal spikes occurred at higher rates in the CA3 area compared to EC (Fig. 4B). Statistical analysis confirmed that there were more type 1 spikes in EC compared to CA3 during the latent phase but more type 1 spikes in CA3 compared to EC during the chronic phase (p < 0.05; asterisk in Fig. 4Ba). Similar to type 1 spikes, type 2 spikes also occurred at higher rates in EC compared to CA3 during the latent phase, but their rate was higher in CA3 compared to EC during the chronic phase (p < 0.05; asterisk in Fig. 4Bb).
Fig. 4.
Rates of occurrence of type 1 and type 2 interictal spikes during the latent and chronic phases. A. Average rates of occurrence of type 1 (a) and type 2 (b) spikes in CA3 and EC at different times before and after the occurrence of the first spontaneous seizure (dotted vertical lines at time 0). B. Over time difference of the rates of occurrence of type 1 (a) and type 2 (b) spikes between EC and CA3 (EC-CA3). Note that overall, rates of occurrence of type 1 and type 2 spikes are higher in EC compared to CA3 during the latent phase (−48 h to −2 h) compared to the chronic phase (+2 to +96 h) (*p < 0.05 in both cases). C. Bar graph showing the average duration of the spike and wave components of type 1 interictal spikes and of the spike component of type 2 interictal spikes during the latent and the chronic phase. Note the significant decrease in duration of type 2 spikes in both CA3 and EC from the latent to the chronic phase (*p < 0.05).
Finally, in order to test for any individual variability of our results, we analyzed in each animal the changes in occurrence of type 1 and type 2 spikes in CA3 and EC. We found that 67% of animals showed an increase in occurrence of type 1 and type 2 spikes in CA3 from the latent to the chronic phase. In the EC, type 1 spikes decreased in occurrence from the latent to the chronic phase in 71% of animals while in 86% of them type 2 spikes decreased in occurrence from the latent to the chronic phase. Fig. 4C summarizes the average duration of the spike and wave components of type 1 spikes and the average duration of type 2 spikes in CA3 and EC during the latent and chronic phases. We found that there was no difference in the duration of the spike or of the wave component of type 1 spikes between the latent and chronic phases in CA3 or EC. In contrast, the duration of type 2 spikes decreased significantly in CA3 and EC from the latent to the chronic phase (p < 0.05).
Type 1 and type 2 spikes co-occurring with high-frequency oscillations
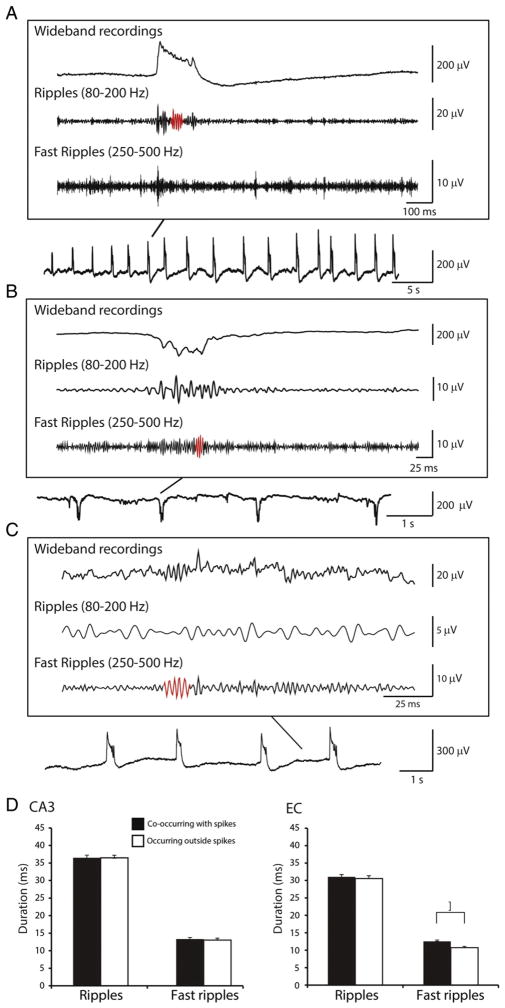
It has been reported in epileptic patients that HFOs can co-occur with interictal spikes or independently of interictal spikes (Jacobs et al., 2008; Zijlmans et al., 2009). We first performed an analysis of the whole recordings, independently of the time of seizure occurrence. As illustrated in Fig. 5, we found that in pilocarpine-treated epileptic rats both ripples and fast ripples occurred during both type 1 and type 2 spikes (panels A and B) as well as outside of interictal spikes (panel C). Analysis of the duration of ripples and fast ripples co-occurring with spikes or occurring outside of spikes revealed no significant differences between them in CA3 (Fig. 5D). There was also no significant difference in duration of ripples co-occurring with spikes and occurring outside of spikes in EC (Fig. 5D). However, fast ripples occurring outside of spikes were significantly shorter compared to fast ripples co-occurring with spikes in EC (p < 0.05; asterisk in Fig. 5D).
Fig. 5.
HFOs co-occurring with type 1 and type 2 spikes as well as outside of them. A. Example of a ripple co-occurring with a type 1 spike in the CA3 region of a pilocarpine-treated rat one day before the occurrence of the first spontaneous seizure. B. Example of a fast ripple co-occurring with a type 2 spike in the CA3 region of the same animal one day after the occurrence of the first seizure. C. Example of a fast ripple occurring in EC outside of interictal spikes on the day of the occurrence of the first seizure. HFOs in A, B and C are highlighted in red. D. Bar graph showing the average duration of ripples and fast ripples co-occurring with spikes and outside of spikes in CA3 and EC. Note that fast ripples occurring outside of spikes were significantly shorter in duration in EC (*p < 0.05).
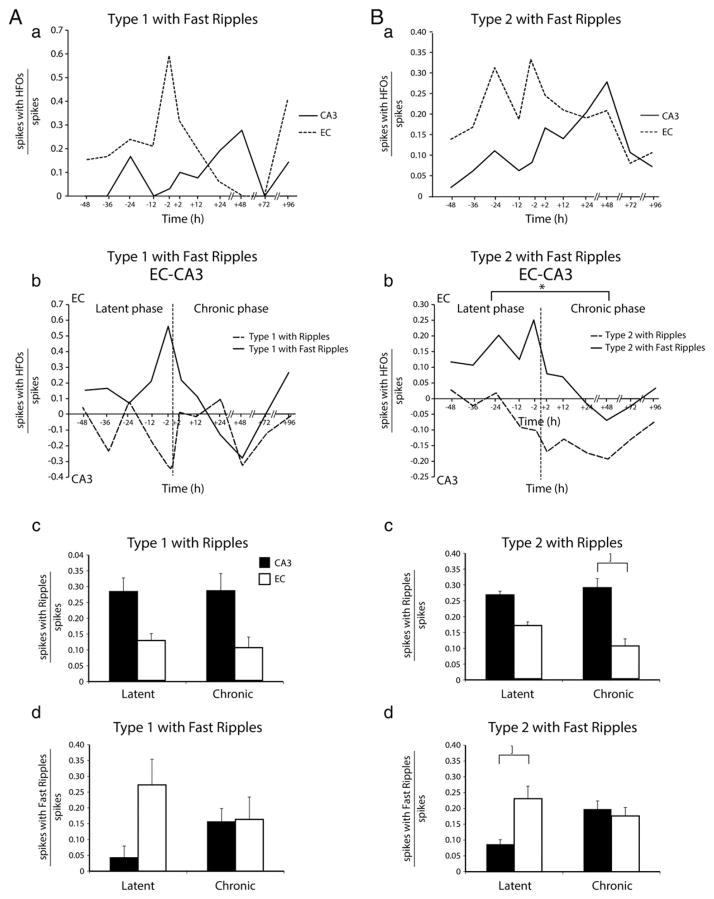
Next, we analyzed the rates of occurrence of type 1 and type 2 spikes that were associated with ripples and fast ripples in CA3 and EC between 48 h before and 96 h after the first seizure (Fig. 6). No significant differences between the latent and chronic phases were found when the rates of type 1 spikes with either ripples or fast ripples were analyzed in EC and CA3 (Fig. 6Aa). The difference of the occurrence of type 1 interictal spikes between EC and CA3 did not show any significant difference between latent and chronic phases (Fig. 6Ab). In addition, the average occurrence of type 1 spikes with ripples (Fig. 6Ac) or fast ripples (Fig. 6Ad) did not differ significantly between latent and chronic phases for both regions. In contrast, as illustrated in Fig. 6Bb, the difference between the rates of type 2 spikes with fast ripples in EC and CA3 was higher during the latent phase compared to the chronic phase (p < 0.05) (absolute values in Fig. 6Ba). It was also found that this difference reached its maximum 2 h before the first seizure (Fig. 6Ba). Overall, the average rate of occurrence of type 2 spikes with ripples was higher in CA3 than in EC during the chronic phase (p < 0.05; asterisk in Fig. 6Bc) while the average rate of occurrence of type 2 spikes with fast ripples was higher in EC compared to CA3 during the latent phase (p < 0.05; asterisk in Fig. 6Bd).
Fig. 6.
Rates of occurrence of type 1 and type 2 interictal spikes co-occurring with HFOs. Aa. Temporal changes of the ratio of occurrence of type 1 spikes with fast ripples. (The absolute values of the ratio of the type 1 spikes with ripples are not shown.) In this plot as well as in that shown in Ba, the ratio in each region was calculated by dividing the number of interictal spikes with ripples or fast ripples over the total number of interictal spikes. Note that there was no significant difference between the latent (−48 h to −2 h) and chronic phases (+2 h to +96 h). Ab. Over time differences between EC and CA3 of the ratios of type 1 spikes with ripples (dotted line) and fast ripples (continuous line). However, the difference between EC and CA3 reached its maximum value 2 h before the occurrence of the first seizure. The inset shows ratios of type 1 spikes with fast ripples in CA3 and EC. Note again the peak at 2 h before first seizure onset. Ac. Average ratios of type 1 spikes with ripples in CA3 and EC during latent and chronic phases. No significant differences were observed. Ad. Average ratios of type 1 spikes with fast ripples in CA3 and EC during latent and chronic phases. No significant differences were observed. Ba. Temporal changes of the ratio of occurrence of type 2 spikes with fast ripples. (The absolute values of the ratio of the type 2 spikes with ripples are not shown.) Bb. Difference over time between ratios of type 2 spikes with ripples and fast ripples between EC and CA3. Note that the difference between EC and CA3 for type 2 spikes with fast ripples is significantly higher in the latent phase compared to the chronic phase (*p < 0.05) and it reaches its maximum value 2 h before the occurrence of the first seizure. Bc. Average ratio of type 2 spikes with ripples in CA3 and EC during latent and chronic phases. Type 2 spikes with ripples occurred at higher rates in CA3 compared to EC during the chronic phase. Bd. Average ratios of type 2 spikes with fast ripples in CA3 and EC during both latent and chronic phases. Type 2 spikes with fast ripples occurred at higher rates in EC compared to CA3 during the latent phase (*p < 0.05).
High-frequency oscillations occurring outside of interictal spikes
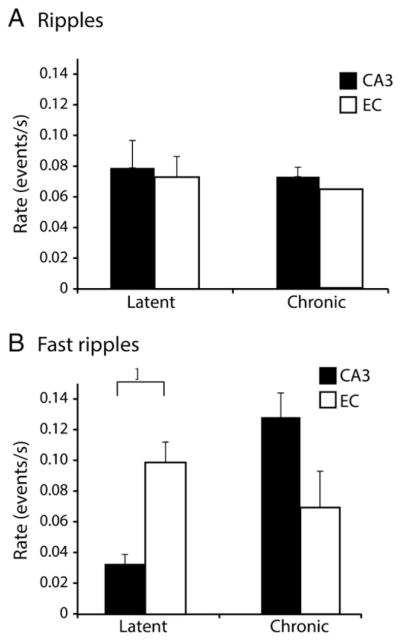
Finally, we analyzed the rate of ripples and fast ripples occurring outside of interictal spikes during the latent and chronic phases. Statistical analyses revealed that during the latent phase ripples outside of spikes occurred at similar rates in CA3 and EC (Fig. 7A) while fast ripples outside of spikes had significantly higher rates in EC than in CA3 (p < 0.05; asterisk in Fig. 6B). In contrast, during the chronic phase the rate of fast ripples outside of spikes was higher in CA3 compared to EC (but marginally significant, p = 0.06; Fig. 7B). Rates of ripples or fast ripples outside of spikes did not show any specific pattern at the transition between the latent and the chronic phase (data not shown).
Fig. 7.
Average rates of occurrence of HFOs outside of spikes during the latent and chronic phases in CA3 and EC. A. Average rates of occurrence of ripples occurring outside of spikes in CA3 and EC during both latent and chronic phases. No significant differences were observed. B. Average rates of occurrence of fast ripples occurring outside of spikes in CA3 and EC during latent and chronic phases. Fast ripples outside of spikes occurred at higher rates in EC compared to CA3 during the latent phase (p < 0.05). On the other hand during the chronic phase the average rate of occurrence of fast ripples is higher in CA3 compared to EC (p = 0.06).
Discussion
The main findings of our study can be summarized as follows. First, there was a switch in the distribution of both types of interictal spikes before and after the occurrence of the first seizure: during the latent period they prevailed in EC while during the chronic period they predominated in CA3. Second, the duration of type 2 spikes decreased significantly in both regions from the latent to the chronic phase. Third, type 2 spikes associated to fast ripples occurred at higher rate in EC than CA3 during the latent phase whereas they occurred at similar rate in the chronic phase. Finally, rates of fast ripples outside of interictal spikes were higher in EC compared to CA3 during the latent phase.
Structure-specific expression of interictal spikes during the latent and chronic phases
We have found that interictal spike rates in EC and CA3 change over time following pilocarpine-induced status epilepticus. Specifically, we identified a shift from higher interictal spike occurrence in EC to higher interictal spike occurrence in CA3 at the transition from the latent to the chronic phase. These results suggest that modifications in interictal spike expression in these two limbic structures may reflect the dynamics of dysfunctional network activity and interactions that should occur during epileptogenesis. According to our findings, the distribution of interictal spikes switches at the transition between the latent and the chronic phase. Interictal spikes predominate in the hippocampus CA3 subfield as compared to EC during the chronic phase suggesting that more robust changes in excitability and connectivity may occur shortly after status epilepticus (and thus earlier) in the former structure. This view is in line with a previous study in which the EC was found to be hyperexcitable and more vulnerable to neuronal damage shortly after status epilepticus induced by lithium–pilocarpine (André et al., 2007); these investigators found that entorhinal and piriform cortices were the initial structures to exhibit signal changes on MRI scans, as early as 6 h after status epilepticus. Moreover, they reported that the extent of EC damage correlated with the length of the latent phase (which was longer when EC damage was minimal). Finally, according to these experiments, neuronal damage in the hippocampus was delayed (André et al., 2007).
More recently, Bragin et al. (2009) have obtained evidence supporting the involvement of the EC during the early stages of the latent phase and thus, presumably, during epileptogenesis. In this study, which was performed in brain slices obtained from lithium–pilocarpine-treated rats, a high percentage of neurons in the EC layer 5 responded to weak stimulation with polysynaptic burst discharges during the latent phase. These findings were attributed to a depolarizing shift of the IPSP reversal potential consequent to intracellular accumulation of Cl−due to upregulation of the inward transporter NKCC1 and downregulation of the outward transporter KCC2. Therefore, according to these investigators MTLE primarily develops in the EC, and only over time it would involve other limbic areas including the hippocampus (Bragin et al., 2009). The predominance of occurrence of interictal spikes in the hippocampus CA3 area compared to the EC observed after the first spontaneous seizure is also in line with the hypothesis that parahippocampal and hippocampal structures are involved at different time points following the initial insult (André et al., 2007; Bragin et al., 2009). Interestingly, El-Hassar et al. (2007) have reported that synaptic current properties in the CA1 area of pilocarpine-treated rats are remarkably different during the early and late parts of the latent phase as well as when compared to those seen during the chronic phase.
Changes in duration of type 2 interictal spikes during epileptogenesis
Contrary to what was reported by Chauvière et al. (2012), type 1 interictal spikes in our study did not decrease in duration when analyzed during the latent phase and, after the first seizure, during the chronic phase. This discrepancy may be explained by the fact that we did not record from the same hippocampal regions (i.e., the CA1 area). In addition, the decrease in duration of type 1 spikes in the study by Chauvière et al. (2012) was observed over a timescale ranging from 8 days before until the day of first seizure occurrence whereas in our study we restricted our analysis to interictal spikes occurring during the 48 h that preceded the first spontaneous seizure. We have however observed a significant decrease in duration of type 2 spikes from the latent to the chronic phase, in both CA3 and EC. Although the cellular and pharmacological mechanisms underlying type 2 spikes remain undefined, it has been proposed that they arise from the synchronous activity of local networks of excitatory cells (Chauvière et al., 2012; Demont-Guignard et al., 2012). This view is supported by evidence obtained from several in vitro experiments in which short-lasting interictal discharges similar to type 2 spikes were shown to result from enhancement of synaptic excitation due to weakening of inhibition, and to rest on recurrent excitation and regenerative Ca2+ currents leading to the synchronous firing of a large number of principal cells (Dingledine and Gjerstad, 1980; Schwartzkroin and Prince, 1980; Traub and Wong, 1982). A primary role of glutamatergic mechanisms along with synchronous firing has also been documented for the short-lasting interictal spikes recorded from the CA3 area of brain slices superfused with the K+ channel blocker 4-aminopyridine, a pharmacological procedure that enhances both GABAergic and glutamatergic neurotransmission (Avoli and de Curtis, 2011). Therefore, the decreased duration of type 2 spikes from the latent to the chronic phase in both CA3 and EC could reflect progressive changes in neural network synchrony mainly supported by glutamatergic mechanisms.
Type 2 spikes with HFOs may mirror epileptogenesis
The breakdown of type 1 and type 2 interictal spikes into subcategories comprising spikes with ripples or fast ripples has revealed that their rates of occurrence vary over time in both regions. Type 1 spikes with either ripples or fast ripples did not change between latent and chronic phases. In contrast, type 2 spikes with fast ripples occurred more frequently during the latent phase in the EC compared to CA3. These data indicate that type 2 spikes with fast ripples could be a better marker of epileptogenesis than type 1 spikes with HFOs or type 2 spikes with ripples.
The mechanisms underlying pathologic HFOs are still under study but evidence suggests that pathologic HFOs in the ripple band represent population IPSPs generated by principal neurons entrained by synchronously active interneuron networks while fast ripples mirror the synchronous firing of abnormally active principal cells reflecting glutamatergic conductances and being independent of inhibitory neurotransmission (Bragin et al., 1999a,b; Dzhala and Staley, 2004; Foffani et al., 2007; see for review Jefferys et al., 2012a,b). According to this view the high rates of type 2 spikes with fast ripples in EC during the latent phase may reflect a dynamic increase in excitatory activity that will eventually lead to the onset of spontaneous seizures; such conditions would be then shifted to the CA3 area once chronic seizures start to occur.
High-frequency oscillations occurring outside of interictal spikes
Fast ripples occurring outside of spikes had higher rates in EC than in CA3 during the latent phase while the opposite occurred during the chronic epileptic phase (although large, this latter difference was not statistically significant). However, the analysis of their rates of occurrence over time did not show any specific shift at the transition between the latent and chronic phases. These findings suggest that HFOs outside of spikes may be less reliable markers of epileptogenicity compared to HFOs co-occurring with spikes. This is in accordance with clinical studies showing that the co-occurrence of HFOs with interictal spikes is a better marker of seizure onset zones in epileptic patients than HFOs occurring alone (Wang et al., 2013).
Conclusions
Our results demonstrate that progressive structure-dependent changes in limbic excitability follow pilocarpine-induced status epilepticus; specifically, we found that type 2 interictal spikes, type 2 interictal spikes associated to fast ripples, and fast ripples outside of spikes predominate in EC during the latent phase and predominate in CA3 during the chronic phase. More specifically the increase in the rate of occurrence of spikes co-occurring with fast ripples in CA3 during the chronic phase seems to be a better pathological marker than other interictal spikes. We propose that these changes reflect the progressive pathological reorganization of limbic neuronal networks leading to the occurrence of spontaneous seizures. Future studies with single-cell recordings are needed to establish the participation of principal cells and interneurons to these interictal events. Moreover, these findings should be validated by careful studies of the interictal patterns (including spikes and HFOs) recorded with depth electrodes from MTLE patients. Although it is impossible to implement this approach during the latent phase in humans, our experimental results may help understanding the progression of epileptogenicity in cases with a relatively high risk of developing epilepsy such as after a traumatic brain injury.
Acknowledgments
This study was supported by the Canadian Institutes of Health Research (CIHR grants 8109, 74609 and 102710).
Footnotes
Conflicts of interest
None of the authors has any conflict of interest to disclose.
References
- André V, Dubé C, François J, Leroy C, Rigoulot MA, Roch C, Namer IJ, Nehlig A. Pathogenesis and pharmacology of epilepsy in the lithium–pilocarpine model. Epilepsia. 2007;48(Suppl 5):41–47. doi: 10.1111/j.1528-1167.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- Avoli M, de Curtis M. GABAergic synchronization in the limbic system and its role in the generation of epileptiform activity. Prog Neurobiol. 2011;95:104–132. doi: 10.1016/j.pneurobio.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw AP, Jacobs J, LeVan P, Dubeau F, Gotman J. Effect of sleep stage on interictal high-frequency oscillations recorded from depth macroelectrodes in patients with focal epilepsy. Epilepsia. 2009;50:617–628. doi: 10.1111/j.1528-1167.2008.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénar CG, Chauvière L, Bartolomei F, Wendling F. Pitfalls of high-pass filtering for detecting epileptic oscillations: a technical note on “false” ripples. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2010;121:301–310. doi: 10.1016/j.clinph.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Bortel A, Lévesque M, Biagini G, Gotman J, Avoli M. Convulsive status epilepticus duration as determinant for epileptogenesis and interictal discharge generation in the rat limbic system. Neurobiol Dis. 2010;40:478–489. doi: 10.1016/j.nbd.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsáki G. High-frequency oscillations in human brain. Hippocampus. 1999a;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia. 1999b;40:127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- Bragin DE, Sanderson JL, Peterson S, Connor JA, Müller WS. Development of epileptiform excitability in the deep entorhinal cortex after status epilepticus. Eur J Neurosci. 2009;30:611–624. doi: 10.1111/j.1460-9568.2009.06863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvière L, Doublet T, Ghestem A, Siyoucef SS, Wendling F, Huys R, Jirsa V, Bartolomei F, Bernard C. Changes in interictal spike features precede the onset of temporal lobe epilepsy. Ann Neurol. 2012;71:805–814. doi: 10.1002/ana.23549. [DOI] [PubMed] [Google Scholar]
- Demont-Guignard S, Benquet P, Gerber U, Biraben A, Martin B, Wendling F. Distinct hyperexcitability mechanisms underlie fast ripples and epileptic spikes. Ann Neurol. 2012;71:342–352. doi: 10.1002/ana.22610. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Gjerstad L. Reduced inhibition during epileptiform activity in the in vitro hippocampal slice. J Physiol. 1980;305:297–313. doi: 10.1113/jphysiol.1980.sp013364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek FE, Staley KJ. The time course and circuit mechanisms of acquired epileptogenesis. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. National Center for Biotechnology Information, (US); Bethesda (MD): 2012. [PubMed] [Google Scholar]
- Dzhala VI, Staley KJ. Mechanisms of fast ripples in the hippocampus. J Neurosci Off J Soc Neurosci. 2004;24:8896–8906. doi: 10.1523/JNEUROSCI.3112-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hassar L, Milh M, Wendling F, Ferrand N, Esclapez M, Bernard C. Cell domain-dependent changes in the glutamatergic and GABAergic drives during epileptogenesis in the rat CA1 region. J Physiol. 2007;578:193–211. doi: 10.1113/jphysiol.2006.119297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J., Jr Introduction to temporal lobe epilepsy. Epilepsy Res. 1996;26:141–150. doi: 10.1016/s0920-1211(96)00043-5. [DOI] [PubMed] [Google Scholar]
- Foffani G, Uzcategui YG, Gal B, Menendez de la Prida L. Reduced spike-timing reliability correlates with the emergence of fast ripples in the rat epileptic hippocampus. Neuron. 2007;55:930–941. doi: 10.1016/j.neuron.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Gloor P. The Temporal Lobe and Limbic System. Oxford University Press; USA: 1997. [Google Scholar]
- Jacobs J, LeVan P, Chander R, Hall J, Dubeau F, Gotman J. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49:1893–1907. doi: 10.1111/j.1528-1167.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Staba R, Asano E, Otsubo H, Wu JY, Zijlmans M, Mohamed I, Kahane P, Dubeau F, Navarro V, Gotman J. High-frequency oscillations (HFOs) in clinical epilepsy. Prog Neurobiol. 2012;98:302–315. doi: 10.1016/j.pneurobio.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys JGR, Menendez de la Prida L, Wendling F, Bragin A, Avoli M, Timofeev I, Lopes da Silva FH. Mechanisms of physiological and epileptic HFO generation. Prog Neurobiol. 2012a;98:250–264. doi: 10.1016/j.pneurobio.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys JGR, Jiruska P, de Curtis M, Avoli M. Limbic network synchronization and temporal lobe epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. National Center for Biotechnology Information, (US); Bethesda (MD): 2012b. [PubMed] [Google Scholar]
- Jiruska P, Csicsvari J, Powell AD, Fox JE, Chang WC, Vreugdenhil M, Li X, Palus M, Bujan AF, Dearden RW, Jefferys JGR. High-frequency network activity, global increase in neuronal activity, and synchrony expansion precede epileptic seizures in vitro. J Neurosci Off J Soc Neurosci. 2010a;30:5690–5701. doi: 10.1523/JNEUROSCI.0535-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiruska P, Powell AD, Chang WC, Jefferys JGR. Electrographic high-frequency activity and epilepsy. Epilepsy Res. 2010b;89:60–65. doi: 10.1016/j.eplepsyres.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Lévesque M, Bortel A, Gotman J, Avoli M. High-frequency (80–500 Hz) oscillations and epileptogenesis in temporal lobe epilepsy. Neurobiol Dis. 2011;42:231–241. doi: 10.1016/j.nbd.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque M, Salami P, Gotman J, Avoli M. Two seizure-onset types reveal specific patterns of high-frequency oscillations in a model of temporal lobe epilepsy. J Neurosci Off J Soc Neurosci. 2012;32:13264–13272. doi: 10.1523/JNEUROSCI.5086-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BS, Kapur J. A combination of ketamine and diazepam synergistically controls refractory status epilepticus induced by cholinergic stimulation. Epilepsia. 2008;49:248–255. doi: 10.1111/j.1528-1167.2007.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Salami P, Lévesque M, Gotman J, Avoli M. A comparison between automated detection methods of high-frequency oscillations (80–500 Hz) during seizures. J Neurosci Methods. 2012;211:265–271. doi: 10.1016/j.jneumeth.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanova V, Markand ON, Worth R. Clinical characteristics and predictive factors in 98 patients with complex partial seizures treated with temporal resection. Arch Neurol. 1994;51:1008–1013. doi: 10.1001/archneur.1994.00540220054014. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Prince DA. Changes in excitatory and inhibitory synaptic potentials leading to epileptogenic activity. Brain Res. 1980;183:61–76. doi: 10.1016/0006-8993(80)90119-5. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Jhung D, Fried I, Engel J., Jr High-frequency oscillations recorded in human medial temporal lobe during sleep. Ann Neurol. 2004;56:108–115. doi: 10.1002/ana.20164. [DOI] [PubMed] [Google Scholar]
- Toyoda I, Bower MR, Leyva F, Buckmaster PS. Early activation of ventral hippocampus and subiculum during spontaneous seizures in a rat model of temporal lobe epilepsy. J Neurosci Off J Soc Neurosci. 2013;33:11100–11115. doi: 10.1523/JNEUROSCI.0472-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Wong RK. Cellular mechanism of neuronal synchronization in epilepsy. Science. 1982;216:745–747. doi: 10.1126/science.7079735. [DOI] [PubMed] [Google Scholar]
- Urrestarazu E, Chander R, Dubeau F, Gotman J. Interictal high-frequency oscillations (100–500 Hz) in the intracerebral EEG of epileptic patients. Brain J Neurol. 2007;130:2354–2366. doi: 10.1093/brain/awm149. [DOI] [PubMed] [Google Scholar]
- Wang S, Wang IZ, Bulacio JC, Mosher JC, Gonzalez-Martinez J, Alexopoulos AV, Najm IM, So NK. Ripple classification helps to localize the seizure-onset zone in neocortical epilepsy. Epilepsia. 2013;54:370–376. doi: 10.1111/j.1528-1167.2012.03721.x. [DOI] [PubMed] [Google Scholar]
- Zijlmans M, Jacobs J, Zelmann R, Dubeau F, Gotman J. High-frequency oscillations mirror disease activity in patients with epilepsy. Neurology. 2009;72:979–986. doi: 10.1212/01.wnl.0000344402.20334.81. [DOI] [PMC free article] [PubMed] [Google Scholar]