Figure 2. Strategy and methods for the synthesis of paracaseolide A and a truncated (methyl-containing) analogue.
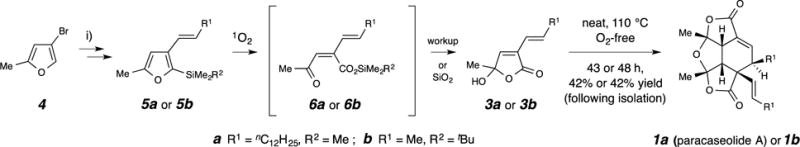
Paracaseolide A (1a) was prepared from 3a3,13,14,15,16 and the truncated analogue 1b from 3b by heating the neat compound in an oxygen-free-atmosphere. The monomers 3a and 3b were prepared by a two-step or four-step sequence, respectively, from 4-bromo-2-methylfuran.
