Figure 4. Conversion of the exo-Diels-Alder product to the natural product.
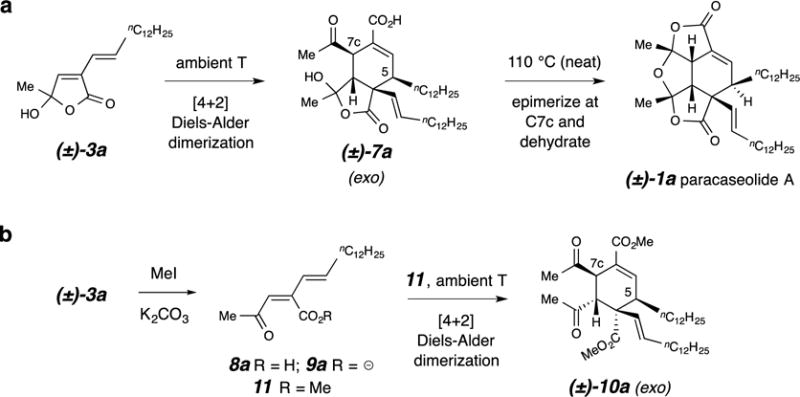
a, Ambient temperature dimerization of butenolide 3a produces a racemate of the exo adduct 7a; thermal dehydration requires additional heating. b, The acyclic methyl ester derivative of 3a, the ketoester 11, also dimerizes in a highly diastereoselective fashion at ambient temperature to produce the exo adduct 10a.
