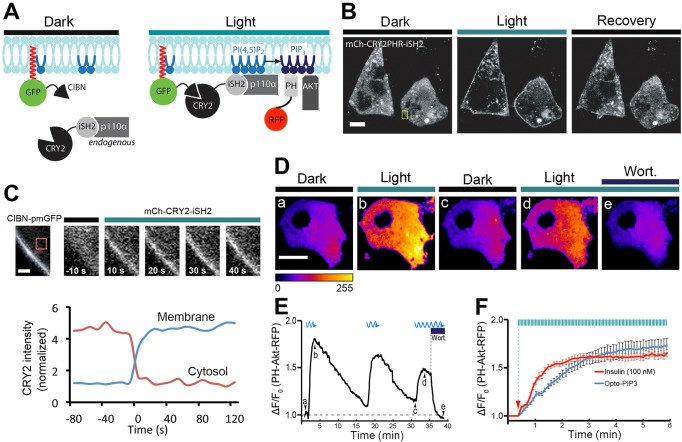
Fig. 1.
Using light to control the PIP3 production in 3T3-L1 adipocytes. 3T3-L1 adipocytes were electroporated with CIBN–pmGFP and mCherry–CRY2–iSH2, or with CIBN–CaaX, CRY2–iSH2 and PH-Akt–mRFP. (A) Schematic of PI3K recruitment to the plasma membrane (CIBN–pmGFP) using a fluorescent CRY2 fusion protein (mCherry–CRY2–iSH2) that constitutively binds to the endogenous PI3K catalytic subunit (p110α). Recruited PI3K converts PIP2 to PIP3 on the plasma membrane, which can be visualized by the PH-Akt–mRFP biosensor. (B,C) Light-triggered translocation of CRY2 fusion protein to the cell surface. Fluorescent images of CRY2 fusion protein were imaged before light activation (dark), 10 s after a 500-ms pulse of blue light (488 nm) and 1 min after pulses of blue light activation (recovery) using spinning disk confocal microscopy (B). Enlarged ROIs (yellow box in B) show the time course of CRY2 fusion protein recruitment to the plasma membrane, and the graph underneath shows quantification of mCherry–CRY2–iSH2 in the cytoplasm and at the cell surface, using the regions shown by the dotted and solid lines, respectively (C). (D–F) Light-induced production of PIP3, its reversibility and sensitivity to PI3K inhibitor wortmannin (wort.). 3T3-L1 adipocytes were activated with 500-ms pulses of blue light (488 nm, 10 mW) at 5-s intervals under TIRFM illumination, and PIP3 production near the cell surface was detected using 561-nm TIRFM laser beam. Selective frames of PH-Akt–mRFP images are shown in D, and corresponding time points (Da–De) are marked in real time fluorescence traces in E. The kinetics of insulin (100 nM) and Opto-PIP3 induced recruitment of PH-Akt–mRFP to the cell surface are plotted in F. Arrow indicates when insulin and blue light were applied. (n=5 cells, data are mean±s.e.m.). Scale bars: 10 μm (B,D); 2 μm (C).