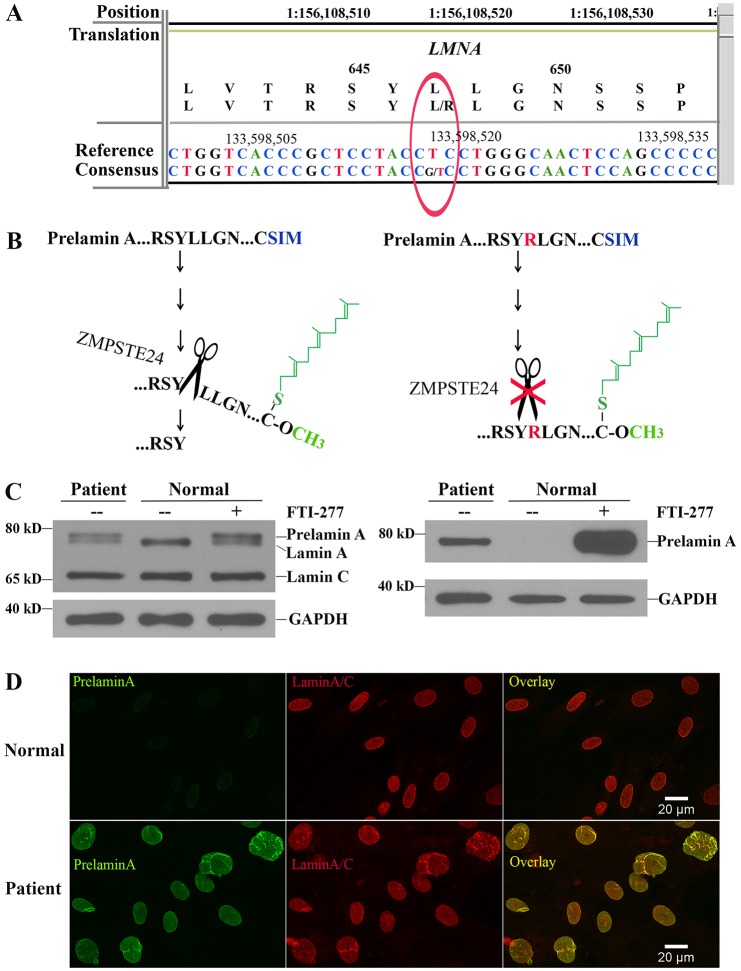
Fig. 2.
Identification of the LMNA L647R mutation predicted to destroy the ZMPSTE24 cleavage site in prelamin A and accumulation of prelamin A in the individual's fibroblasts. (A) Readout of next-generation sequencing and the presence of a heterozygous T>G transversion that results in a L647R amino acid substitution in prelamin A. (B) The L647R amino acid substitution is predicted to abolish the endoproteolytic cleavage of farnesylated-carboxymethylated (represented in green) prelamin A that is catalyzed by ZMPSTE24 (represented by scissors). (C) Immunoblots of protein extracts from fibroblasts of the individual (patient) and fibroblasts from a normal individual (normal) with or without treatment with an FTI (FTI-277). Blots were probed with an anti-lamin A/C antibody that recognized prelamin A, lamin A and lamin C (left) or an antibody that specifically recognized prelamin A (right). Labeling with an antibody against GAPDH is shown as a loading control. Migrations of molecular mass standards are indicated at the left of each blot. (D) Immunofluorescence micrographs of fibroblasts from a normal subject (top panels) and the individual (bottom panels) labeled with antibodies that specifically recognized prelamin A (green), lamin A/C (red) and the overlay of these signals (yellow).