Fig. 1.
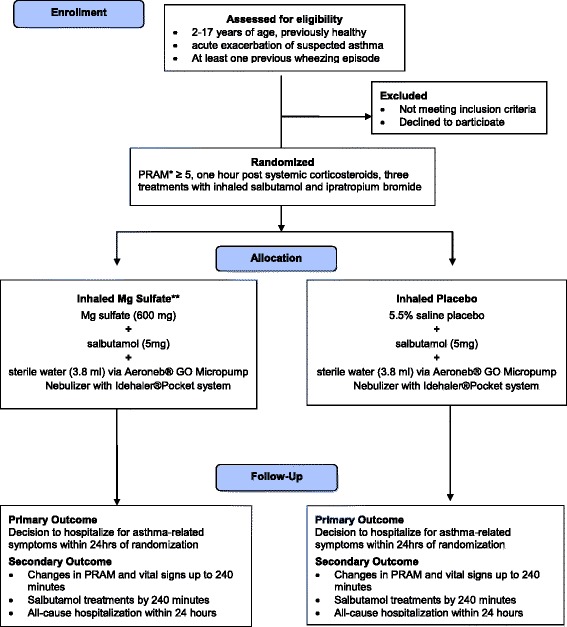
Flow diagram of participant study flow. The intervention consists of two treatment arms with solutions of identical osmolarity consisting of inhaled salbutamol with Mg sulfate (experimental arm) and with equivalent saline placebo (control arm). The primary outcome is hospitalization to inpatient unit for asthma-related symptoms within 24 h of randomization. *PRAM Pediatric Respiratory Assessment, **Mg magnesium
