Figure 1. Acetylation at K305 Decreases PKM2 Enzyme Activity.
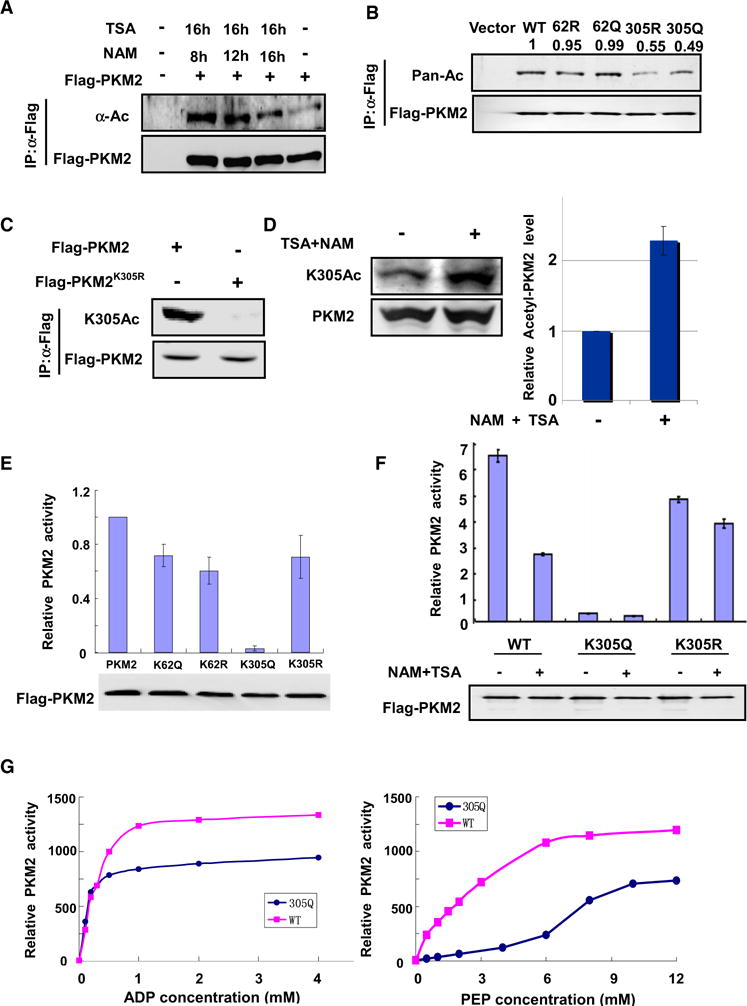
(A) PKM2 is acetylated. Flag-PKM2 was transfected into 293T cells followed by treatment with TSA and NAM for the indicated time, and PKM2 acetylation and protein levels were analyzed by western blot with indicated antibody, respectively.
(B) Mutation of K305 decreases PKM2 acetylation. The indicated plasmids were cotransfected into 293T cells, and protein was immunoprecipitated (IP) for acetylation analysis. Acetylation levels were normalized against β-actin.
(C) Characterization of acetyl-PKM2 (K305) antibody. The indicated plasmids were transfected into 293T cells, and acetylation level of IPed Flag-PKM2 was measured by the site-specific K305 acetylation antibody.
(D) Endogenous PKM2 is acetylated at K305. 293T cells were treated with TSA and NAM. Endogenous PKM2 was immunoprecipitated, and protein levels and acetylation of K305 were determined by western blot with indicated antibodies (left panel). Relative PKM2 K305 acetylation over protein level was quantified (right panel). Error bars represent ± SD for triplicate experiments.
(E) K305Q mutant decreases PKM2 enzyme activity. Flag-tagged wild-type and mutant PKM2 protein were expressed in 293T cells and purified by IP. The enzyme activity was measured and normalized against protein level. Mean values of relative enzyme activity of triplicate experiments with standard deviation (±SD) are presented.
(F) NAM and TSA treatment decreases PKM2 wild-type but not mutant enzyme activity. Flag-tagged wild-type and mutant PKM2 protein were expressed in 293T cells and treated with or without NAM and TSA, then purified by IP. The PKM2 enzyme activity was measured and normalized against protein level. Mean values of relative enzyme activity of triplicate experiments with standard deviation (±SD) are presented.
(G) K305Q mutation decreases the binding affinity toward PEP. The activities of wild-type and mutant PKM2 were assayed with increasing concentrations of ADP or PEP as indicated. Error bars represent ±SD for triplicate experiments.
