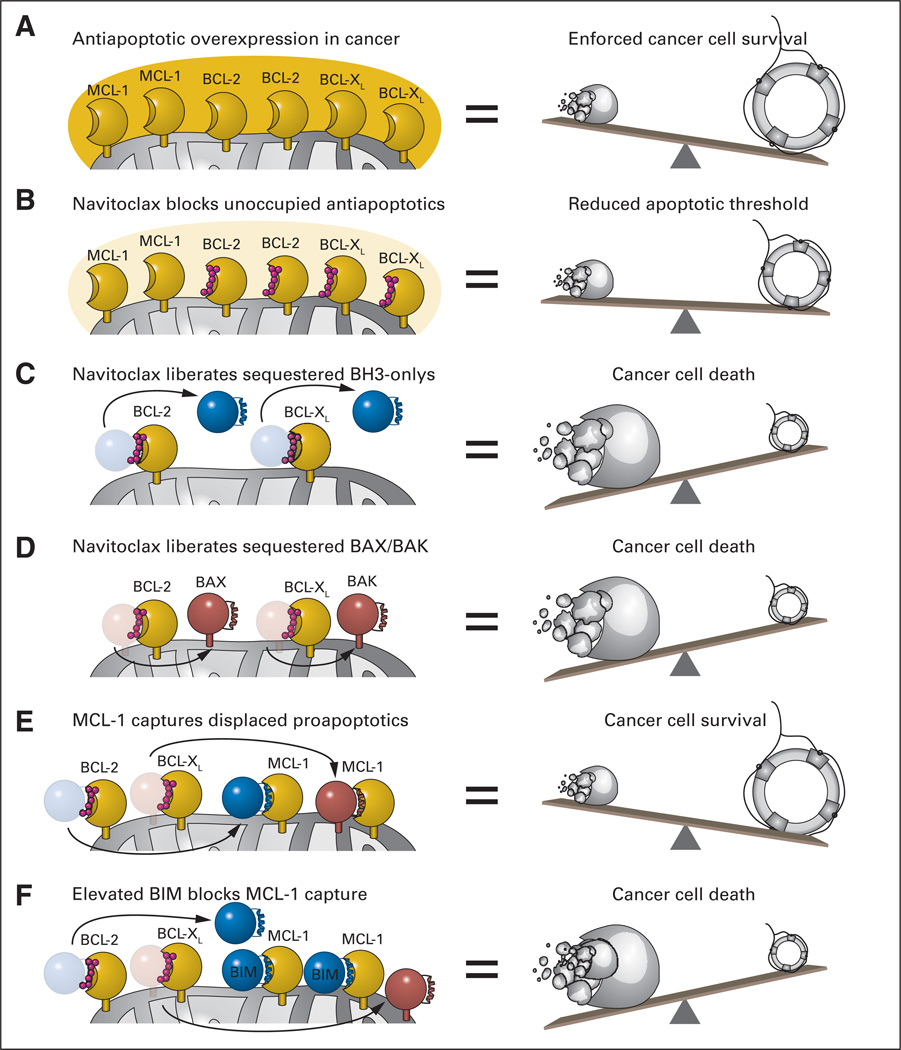
Fig 2.
(A) A common mechanism employed by cancer cells to withstand mitochondrial assault by proapoptotics and thereby ensure pathologic survival is to overload mitochondria with antiapoptotic BCL-2 family proteins. By simulating the natural α-helical BH3 domain that selectively engages the surface groove of antiapoptotic BCL-2 and BCL-XL, navitoclax inhibits the inhibitors of mitochondrial apoptosis. Among its repertoire of proapoptotic activities, navitoclax can (B) lower the threshold for apoptosis by blocking the BH3-binding pockets of unoccupied BCL-2/BCL-XL proteins, (C) displace BCL-2/BCL-XL–sequestered BH3-only proteins to activate BAX/BAK and/or inhibit antiapoptotic proteins not blocked by navitoclax, and (D) competitively release activated forms of BAX/BAK from the heterodimeric lock hold of BCL-2/BCL-XL. (E) Whereas the capacity of navitoclax to potently inhibit BCL-XL leads to unwanted platelet apoptosis, its inability to block a broader spectrum of antiapoptotic proteins, such as myeloid cell leukemia 1 (MCL-1), can account for drug resistance. (F) Such resistance is averted by elevated levels of endogenous BIM, which can mitigate the antiapoptotic activity of MCL-1. Thus, a delicate (yet feasible) pharmacologic balancing act is required to achieve a therapeutic window for navitoclax-induced apoptosis in chronic lymphocytic leukemia.