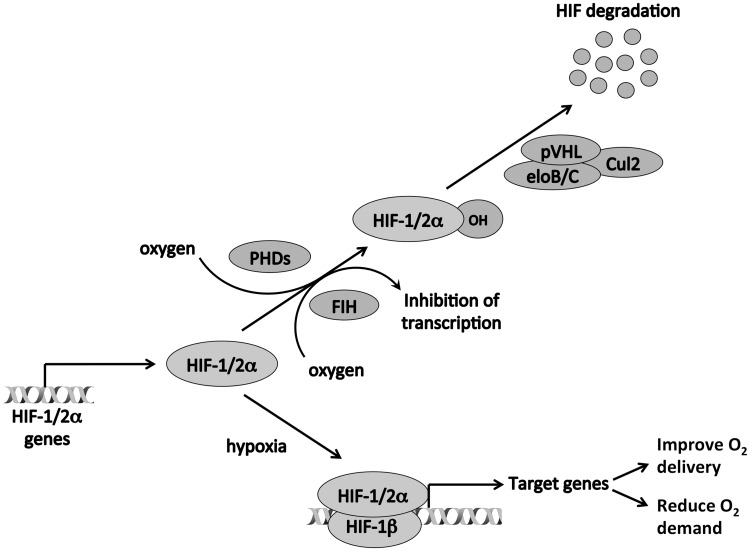
Figure 1.
Regulation of transcription by HIF. In normoxia, HIF-α subunits are hydroxylated by both PHDs and by FIH. HIF-α that has been hydroxylated by the PHDs is recognized by pVHL, ubiquitinated and destroyed in the proteasome. Hydroxylation by FIH blocks the interaction between HIF-α and p300/CBP, inhibiting the transcriptional activity of HIF. In hypoxia, hydroxylation of HIF-α subunits is impaired, leading to their accumulation, dimerization with HIF-1β, binding to HREs and transactivation of target genes.