Summary
This article presents possible applications of ultrasound elastography in musculoskeletal imaging based on the available literature, as well as the possibility of extending indications for the use of elastography in the future. Ultrasound elastography (EUS) is a new method that shows structural changes in tissues following application of physical stress. Elastography techniques have been widely used to assess muscles and tendons in vitro since the early parts of the twentieth century. Only recently with the advent of new technology and creation of highly specialized ultrasound devices, has elastography gained widespread use in numerous applications.
The authors performed a search of the Medline/PubMed databases for original research and reviewed publications on the application of ultrasound elastography for musculoskeletal imaging.
All publications demonstrate possible uses of ultrasound elastography in examinations of the musculoskeletal system. The most widely studied areas include the muscles, tendons and rheumatic diseases. There are also reports on the employment in vessel imaging.
The main limitation of elastography as a technique is above all the variability of applied pressure during imaging, which is operator-dependent. It would therefore be reasonable to provide clear guidelines on the technique applied, as well as clear indications for performing the test. It is important to develop methods for creating artifact-free, closed-loop, compression-decompression cycles.
The main advantages include cost-effectiveness, short duration of the study, non-invasive nature of the procedure, as well as a potentially broader clinical availability. There are no clear guidelines with regard to indications as well as examination techniques.
Ultrasound elastography is a new and still poorly researched method. We conclude, however, that it can be widely used in the examinations of musculoskeletal system. Therefore, it is necessary to conduct large, multi-center studies to determine the methodology, indications and technique of examination.
MeSH Keywords: Elasticity Imaging Techniques; Ultrasonography; Ultrasonography, Doppler, Color
Background
Elastography (EUS) is a new technique complementary to conventional ultrasound and Doppler studies. This technique is based on a general principle that an agent acting on tissues induces changes to those tissues depending on their elastic properties. Thus, it allows for evaluation of an additional, previously unexamined parameter such as elasticity, enabling qualitative and quantitative assessment of mechanical properties of examined tissues independent of acoustic impedance or vascular flow [1–5]. It opens new possibilities in ultrasound studies.
Introduction of elastography to ultrasound studies is a discovery of recent years and another breakthrough following introduction of Doppler techniques. In the previous years a number of clinical studies in this area has significantly increased, i.e. regarding possible applications of this method. Latest publications show that the technique of elastographic examination in conjunction with flow imaging enables assessment of both morphology and, in part, function of examined tissues or organs. It ensues from the sensitivity of elastography with regard to structural changes accompanying physiological as well as pathological processes [6].
First studies utilizing elastography for medical purposes were conducted in 1970 and 1980 in Marsden Royal Hospital, Great Britain, by Kit Hill. Hill’s earlier experience gained in the military engendered an idea regarding assessment of tissue motion on the basis of various ultrasound features. In his monograph Hill described types of motion that are also utilized in the current elastographic studies. According to his classification, we may distinguish four types of tissue motion. Primary motion, such as movement of myocardial walls or the fetus, secondary – e.g. hepatic movement in response to vascular action, tertiary motions include movement of fluids, e.g. blood, and quaternary – elicited by an external force. Hill considered the secondary motions and movements elicited by external mechanical force most interesting. The above-mentioned effects may reveal the mechanical properties of tissues, such as compressibility or firmness. The author also introduced a concept of “remote palpation” and defined it as a tissue response to external acoustic force [7,8]. Subsequent years brought medical reports on the use of elastography in differentiation between normal liver parenchyma and metastatic lesions.
Major development of ultrasonographic techniques and devices for imaging of tissue elasticity began at the end of the 1980’s. Almost every feature of current ultrasound imaging of tissue elasticity roots from research studies conducted in this period. Beginning from the end of 1990’s various laboratories developed alternative methods of examining tissue elasticity using force generated by an acoustic wave [6].
Assuming that tissue damage causes structural abnormalities and changes to physical properties, which translate into changes in elasticity, it may be concluded that such disturbances should appear in elastographic imaging. Despite wide use of EUS techniques for assessment of muscles and tendons in in vitro studies from the beginning of 1990’s [9], only modern technology and development of highly specialized ultrasound devices enabled its widespread application [10–30].
Elastography Techniques
There are several elastography techniques currently used in clinical practice. The fundamental difference between them involves the type or source of applied pressure. Another difference ensues from various methods of detecting displacement of the examined structures. Basic techniques include: strain EUS, shear wave EUS, transient EUS and acoustic radiation force impulse EUS (ARFI EUS) [2,3,10]. It should be noted that diverse nomenclature used by the medical device companies might be confusing. Strain EUS also known as relative strain elastography, sonoelastography, or real-time elastography is the most commonly used technique among the above-mentioned modalities [12–18]. This technique is currently used i.e. in examination of breast tissue.
Strain EUS technique involves placing a transducer over the examined structure. It is important that the transducer is located exactly perpendicular to the studied object. Transducer anisotropy or displacement should be avoided during the examination. Present apparatuses allow transverse displacement not exceeding 4 mm. After placing the transducer over the examined structure, steady, rhythmical compressions (not exceeding 2 mm) begin at a frequency of 1–2/second. The main principle of this technique involves obtaining axial deformation in response to compressions, which is subsequently analyzed in both phases – before and after the compression [4]. The entire technique is based on Hooke’s law and determination of Young’s elastic modulus.
Hooke’s law is a mechanical law describing the dependence of strain on the stress applied to the object. It says that deformation of a body due to the force acting on it is directly proportional to this force. The coefficient determining relationship between force and strain is known as the elasticity modulus. On the assumption that force is applied uniformly, elasticity modulus is inversely proportional to measured deformation. Strain elastography involves measurements of relative strain in one area in relation to another. Appropriate maps, or elastograms, are developed based on that basis. Since the pressure is exerted manually, pictures of acquired strain may differ significantly depending on applied pressure as well as depth of examined tissues or position of the transducer. Thus, the result depends largely on the examiner. Advanced methods of signal processing aimed at minimizing influence of the above limitations [1–3]. In currently used devices strain data are superimposed on the image in B presentation. Such a juxtaposition produces a map – an elastogram. Color-coded maps are encountered most frequently, although it is possible to generate maps only composed of the shades of gray.
It is widely accepted that red color signifies soft tissues, blue – firm/rigid, and yellow/green corresponds to tissues of moderate stiffness. Information acquired with the help of this method may be qualitative or semiquantitative [2,3]. Strain in a given area may be only analyzed in comparison to the rest of the elastogram. Thus, the acquired image is relative. Said method of semiquantitative measurements involves determining the proportion of strain in the area of interest to the strain produced in reference tissue (usually fat tissue).
Elastography is widespread in some areas of medicine. It is most often used in oncology, where it is utilized for imaging of lesions in the liver, breasts, or prostate [31–37]. It is also used in thyroid gland imaging, gynecological studies, or examination of the musculoskeletal system [10–30].
Acoustic radiation force impulse (ARFI) is a type of strain elastography. This technique does not use mechanical pressure. Tissues are stimulated from the inside through a strong, focused acoustic impulse [10,38–40]. In response to this impulse soft tissues undergo greater deformation compared with hard tissues. When the impulse ceases tissues regain their previous dimensions. This technique allows for creating qualitative, black-and-white or color elastograms. This method is used for imaging of deeper structures that cannot be visualized with strain EUS. It is utilized in liver, thyroid and breast imaging [41].
Shear wave EUS is based on entirely different physical principles. Shear waves are formed within the tissue as they interact with conventional ultrasound waves produced by the transducer. Shear waves diverge perpendicularly to axial displacement caused by the ultrasound impulse [42]. Shear wave velocity can be calculated and used for assessment of tissue firmness, simultaneously computing Young’s module of elasticity according to the following equation:
where: E – Young’s module in Kpa, V – shear wave velocity in cm/s [42].
This technique enables acquisition of both color-coded qualitative elastograms, as well as quantitative maps of elasticity or wave velocity. Due to lack of strain and quantitative character of the data this method is considered more objective than strain elastography. This technique is meant for assessment of deep tissues. Appropriate ultrasound penetration depth is necessary for the wave to form [10,42].
Transient EUS, also known as vibration-controlled transient elastography, is a variant of shear EUS [43]. This method is based on the assessment of the velocity of shear wave propagation. The difference is such that the detection wave is sent as in a pulsatile manner in order to avoid error caused by a rebound phenomenon or wave superposition on the tissue border. It allows for differentiation between waves produced intentionally from the reflected ones [43]. Such a type of elastography is focused for liver examination.
Elastography in Tendon Examination
Elastographic examination of tendons is a topic of numerous scientific publications (Figures 1, 2). The main area of interest is examination of the Achilles tendon (Figures 3, 4), which is perfect for application of this technique. This tendon is located superficially and surrounding tissues differ with regard to elasticity. In one of the studies conducted on 50 healthy, asymptomatic volunteers there were two elastographic image patterns of the Achilles tendon [13]. The first pattern showed Achilles tendon as a uniformly firm structure (blue), while the other, more frequent (62%) pattern presented it as a heterogeneous tissue with interwoven longitudinal or spindle-shaped soft tissue strands. Also, elastographic picture did not correspond to the examination in B projection or a Doppler study [13]. The majority of healthy tendons were firm (86–93%). Moderately soft (yellow) tendons were observed in 7–12% of cases, while distinctly soft (red) in 0–1.3% of subjects. On the other hand, in the majority of cases pathological tendons contained distinctly (57%) or moderately (11%) soft areas. Hard structures were noted in 32% of patients [28]. Tissues that appear moderately soft in EUS did not deviate from normal in B-mode examination, while the tendons with distinctly increased compliance (red) [14–16] usually showed changes in classic USS. Thus, the authors suggested that only the tendons denoted as “soft” in EUS should be examined with respect to pathology [15,16]. However, it is still unclear how such images should be interpreted in asymptomatic patients without changes in classic ultrasound examination. It is thought that they might indicate preclinical changes or constitute false positive cases ensuing from tissue displacement on the border of collagen fibers [13–16]. Further research, including MRI, is needed. It is also necessary to compare the results with histopathological examination.
Figure 1.
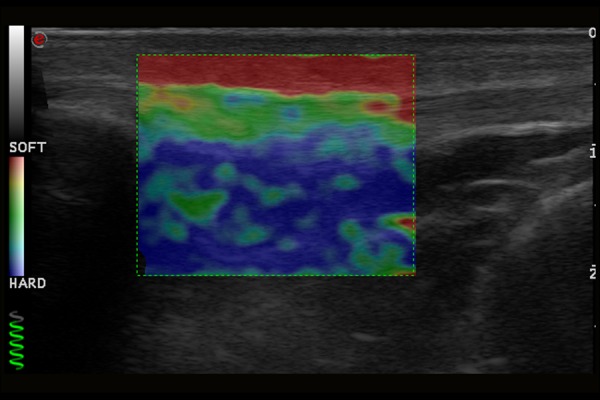
Normal image of patellar ligament in ultrasound elastography examination.
Figure 2.
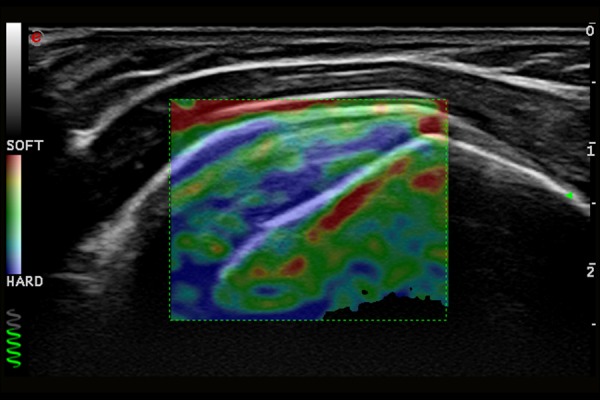
Normal image of supraspinatus muscle tendon in ultrasound elastography examination.
Figure 3.
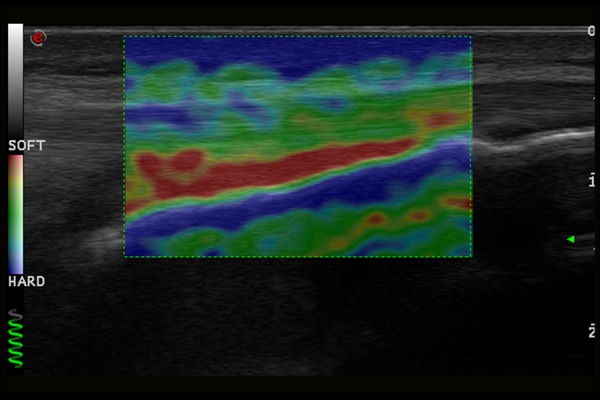
Normal image of Achilles tendon in ultrasound elastography examination.
Figure 4.
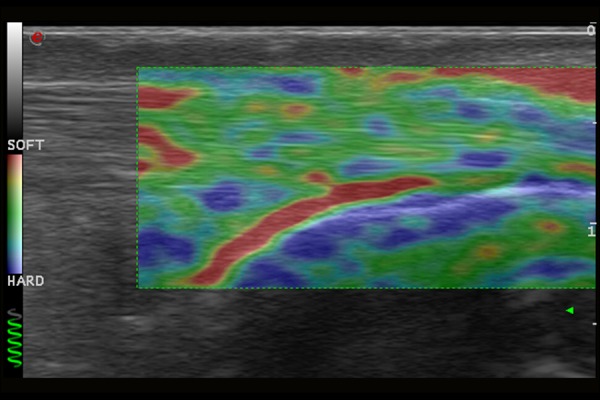
Normal image of Achilles tendon in ultrasound elastography examination.
Different results were obtained in a study analyzing images of abnormal Achilles tendons in 12 athletes. Based on a comparison of USS and magnetic resonance imaging studies with strain elastography it was determined that abnormal tendons exhibit increased stiffness. Due to discrepancies between results further research needs to be conducted in this case as well [13–17].
Assuming that yellow areas correspond to a normal tendon and red regions indicate pathological changes concordance with regard to classic USS reaches 97%. In comparison to clinical examination sensitivity of EUS reaches 93.7% and specificity 99.2% [14]. Both intra- and interobserver repeatability of EUS are very high in case of qualitative study [13–17], but unsatisfactory (20–37%) in case of semiquantitative methods [13].
Beside assessment of the Achilles tendon, the usefulness of elastography in the diagnostics of enthesopathy of the lateral epicondyle of humerus was also evaluated. Significantly reduced stiffness of an abnormal extensor tendon correlating with ultrasound and clinical picture was also revealed [18]. EUS examination visualized more focal lesions and more frequent involvement of lateral tendon or fascia than the traditional ultrasound scan. It was also characterized by higher accuracy than USS and better correlation with the clinical picture [18].
Some publications indicate possible role of strain elastography in the diagnostics of injuries or degenerative diseases of muscles and tendons [44–46]. Despite the existence of studies regarding use of shear wave elastography in the assessment of muscle and tendon stiffness, they have not been yet accepted in clinical practice. There was also a study published on elastographic assessment of master muscle, gastrocnemius muscle and supraspinatus muscle among healthy volunteers [44]. Moreover, preliminary data on the use of shear wave elastography indicate that a healthy Achilles tendon and patellar tendon are characterized by homogeneous hardness and less changes than in strain elastography images [41]. In our opinion it is necessary to create reference elastograms in order to enable comparison with pathological structures.
Elastography in Muscle Assessment
Literature contains isolated publications describing use of strain elastography in the diagnostics of skeletal muscle. In a study focused on EUS and biochemical assessment of healthy muscle during physical exertion it was demonstrated that strain EUS is appropriate for obtaining maps of muscle elasticity [19]. There are also reports demonstrating differences in elasticity of masseter or eye muscles (strain index) depending on sex. Another study assessed elasticity of medial rectus muscle depending on a position of the eye [20,21]. However, precise, large-population data on the analysis of healthy muscles is lacking.
Elastograms show that normal muscle in a resting phase is heterogeneous (mosaic of colors), characterized by intermediate or somewhat increased firmness (respectively: green/yellow or blue), with dispersed areas of reduced and increased firmness, particularly at the peripheries [22,23]. There are no studies comparing the relationship between colors and histopathological changes and causes of the above-described heterogeneity are still unknown.
Strain elastography was also used to study degenerative and neuromuscular diseases. Examination of inflamed muscles revealed changes in elasticity as well as increased firmness in the presence of fibrosis and reduced firmness in the presence of greater amounts of fat. Since a relationship between quantitative parameters of EUS and elevated serum inflammatory markers has been established, it was concluded that EUS might be helpful in evaluation of advancement and monitoring of inflammatory myopathies [22]. Interesting results were obtained in a patient with Bethlem’s myopathy, in whom a correlation was demonstrated between EUS imaging, USS and MRI. Moreover, EUS showed changes undetectable in classical USS or MRI, which may indicate greater sensitivity of this study in examination of such pathologies [23].
Study utilizing propagation of vibrations and Doppler signal suggests that EUS might be useful in selection of musculofascial trigger points as targets for rehabilitation [24].
It is also worth reporting a series of clinical cases of children with spasticity due to cerebral palsy, where EUS enabled creation of elasticity maps in spastic muscles as well as identification of optimal injection sites for administration of botulin toxin therapy [25].
Presented data indicate that EUS might be useful in early diagnosis and evaluation of skeletal muscle disorders [48–50].
Elastography in Rheumatic Disease
There is little data on the use of EUS in inflammatory diseases [26–30]. There was a case study report of a patient with polymyalgia rheumatic: elastographic study revealed bursitis in the region of supraspinatus tendon, which constituted one of the symptoms of the disease. The lesion was undetectable in B-mode projection [26]. Study conducted among patients with systemic scleroderma demonstrated the usefulness of elastography in imaging of skin lesions (reduced skin compliance on the forearm) over the course of this disease [28]. A number of other studies showed that EUS is helpful in the differential diagnostics of rheumatoid nodules and tophi, where rheumatoid nodules were characterized by significantly reduced elasticity [29]. These reports indicate that elastography enables visualization of lesions and may expand its applications in the diagnostics of rheumatic diseases in the future. EUS is also used in the diagnostics of synovitis in a course of rheumatoid arthritis or the diagnostics of articular tuberculosis [11].
Importantly, the majority of the above analyses were conducted on a small number of patients. It is necessary to conduct further, multicenter research studies in order to gain better understanding and confirm the effectiveness of the modality in question [41].
Thrombosis
Venous Doppler examination is a test associated with musculoskeletal system. When venous thrombosis is identified it is important to estimate age of the thrombus. Older thrombi that adhere to vascular wall do not pose a great threat of pulmonary embolization, while “fresh” thrombi significantly increase the risk of such complication. Time from thrombus formation may be often estimated based on the appearance of the skin or patient’s history. Dilated, hypoechogenic vessel lumen is an ultrasonographic determinant of fresh thrombosis. Problems appear when morphology of the thrombus changes and vessel lumen is within normal limits. In such situation differentiation between acute and subacute thrombosis becomes much more difficult and such cases justify the use of elastography in order to precisely determine the age of the thrombus.
Elastography was also used in an experimental study on a dog model aimed at estimating thrombus stiffness in aneurysms at different stages of their formation [51].
Technical Aspects and Limitations of EUS
Diversity of EUS techniques and management algorithms is one of the main problems of elastography. For that reason, results of examination that are influenced by artifacts and other limitations of a given modality might be specific to a particular ultrasound machine. The majority of technical problems and their solutions were identified through analysis of manual EUS examinations. This method constitutes a technical challenge. Formation of artifact-free, closed loops of compression-decompression cycles and application of appropriate, moderate compressing force poses a challenge. Application of too much or too little force will cause the elastic properties of tissues to become non-linear, which will affect calculations of strain [31].
The majority of currently manufactured EUS systems are equipped with software that uses a marker (a green strand or a spring displayed on the screen) to provide information regarding applied force. In order to minimize disparities between assessments of elastograms, measurements should be based on the analysis of entire memory loops of elastograms, not only single pictures [13,16,20]. The most commonly used method for assessing representative images coming from memory loops involves evaluation of at least three compression-decompression cycles, while all of these images should come from the middle phase of each compression cycle. Measurements based on images acquired at the initiation or at the end of compression will be inaccurate [14–16]. In light of the described problems it is necessary to conduct further analyses aimed at creating broad and clear instructions for proper examination technique.
Another significant problem of EUS involves inability to conduct quantitative measurements, which necessitates use of alternative methods, such as semiquantitative measurements (strain ratio) [13], qualitative elastogram assessment using equations, scoring systems and gradation [13–16] or help of external software [21,22]. The above limitations cause problems with interpretation and repeatability of results, or difficulty comparing results of various studies even when performed with the same technique.
It should be remembered during each study that EUS technique is different from B-mode and is governed by different laws [14–16]. In a standard USS tendons should be visualized in transverse and longitudinal planes. In strain EUS longitudinal cross-sections are characterized by the best image quality. Reduced repeatability of measurements acquired in transverse cross-sections ensues from artifacts in lateral parts of the image due to non-homogeneous distribution of pressure on the examined surface. However, this problem can only be solved through superposition of several EUS images – that is, examining entire loops. Moreover, due to difficulty applying uniform force, assessment of superficial, protruding structures as well as elevated areas adjacent to bony structures (e.g. at the level of lateral malleolus during assessment of posterior tibial and fibular muscle tendons) is also hindered.
When comparing elastograms one should also take their size into consideration. As previously mentioned, elastogram is a picture of relative deformations, where elasticity of the examined structure is compared with that of adjacent tissues. Thus, firmness of surrounding tissues will affect the image of examined tissue. It is not a problem in the assessment of breast lesions, where the surrounding tissues are homogeneous (fat, gland tissue). However, when EUS is used for the diagnostics of musculoskeletal system elastogram may encompass tissues of various elastic properties (fat, tendon, bone and muscle), contributing to broad dispersion of acquired data. For the Achilles tendon suggested standard imaging dimension encompasses depth up to three times the thickness of a tendon and length of about ¾ of the screen [16]. However, these criteria are not always applied, making it more difficult to compare the results.
Another problem associated with test standardization is the distance between a transducer and studied tissue. During assessment of many musculoskeletal structures (including Achilles tendon) the area of interest is located superficially. In most ultrasound machines equipped with elastography option there is a need for maintaining a minimum distance (usually 1–2 mm) between examined structure and surface of the skin. Thus, gel pad spacers should be used when examining thin patients in order to increase the distance between skin surface and ultrasound transducer [14–17].
Conventional imaging of musculoskeletal system often requires use of copious amounts of gel in order to smoothen the surface and reduce tissue compression. Amount of gel in the studied area may however influence the elasticity of studied tissues, showing tendons as structures of greater firmness compared to the gel itself.
One should also keep in mind several artifacts that may be revealed during elastography of musculoskeletal system and lead to erroneous interpretation of acquired images. Tendon margins are at particular risk of their formation; red (soft) lines around calcifications are visible as well as the areas behind the bone and on the surface border of homogeneous lesions [16–30]. Similar changes are observed in the intertissue space between neighboring muscles, which is related to tissue movement. In case of cystic changes artifacts are visualized as a mosaic of color. Artifacts are also formed when lesions are located adjacent to large vessels due to pulsation transmitted from those vessels [30].
USS Elastography Perspectives
Elastography constitutes probably the most important technological breakthrough in the field of ultrasonography since development of Doppler imaging. It is characterized by numerous advantages compared to other tests assessing estimated tissue elasticity, such as magnetic resonance elastography. Advantages include low cost, short examination time, noninvasiveness, and possibly broader clinical accessibility. Results of studies conducted to date indicate that EUS may be used for assessment of mechanical properties of musculoskeletal structures under clinical conditions. Moreover, it may be more effective than MRI and gray-scale USS in detection of subclinical muscle and tendon disorders. Due to the above reasons EUS could be applied in early diagnostics and monitoring of physiotherapy, or as a research tool in assessment of biochemical and pathophysiological changes taking place in the course of musculoskeletal diseases [41].
However, despite great interest in EUS available literature is still limited and focuses mainly on case studies and small-population studies without control groups, utilizing various techniques and scoring systems. There are also technical issues in play, such as lack of methods for quantitative analysis, presence of artifacts, or observer-dependent factors that limit repeatability of the test [41].
Doubts with respect to clinical usefulness of EUS stem from the fact that most visualized lesions were already visible in conventional USS or Doppler imaging. There were also reports of lesions detected in EUS, which were not confirmed in other imaging studies and turned out to be clinically insignificant.
In view of these controversies soft tissue elastography requires systematic analysis and standardization based on the suggestions of producers and an agreement between operators with respect to such factors as: equipment, examined parameters, scoring systems, etc. These actions should ensure test repeatability and comparison of results. Because of difficulties associated with the use of EUS for examination of superficial tissues cooperation between manufacturers and scientists should contribute to development of study protocols dedicated to assessment of musculoskeletal system [41].
Another necessary step involves establishing indications for EUS. They should be focused on symptomatic patients with lesions undetectable in standard USS as well as patients at early stages of disease. Comparison of elastography with conventional imaging is also necessary. Multicenter, long-term research studies encompassing large, diverse populations with appropriately long follow-up times need to be conducted. It is also important to compare acquired data with conventional examinations (USS, MRI) and histological studies, as well as to correlate them with the clinical picture. Strain elastography should be compared with modern techniques enabling quantitative assessment, such as shear wave elastography or acoustic radiation force impulse elastography (ARFI) [41].
This review paper precedes our report based on own material gathered among patients examined with US elastography technique.
References
- 1.Hall TJ. AAPM/RSNA physics tutorial for residents: topics in US: Beyond the basics: elasticity imaging with US. Radiographics. 2003;23:1657–71. doi: 10.1148/rg.236035163. [DOI] [PubMed] [Google Scholar]
- 2.Garra BS. Imaging and estimation of tissue elasticity by ultrasound. Ultrasound Q. 2007;23:255–68. doi: 10.1097/ruq.0b013e31815b7ed6. [DOI] [PubMed] [Google Scholar]
- 3.Garra BS. Elastography: current status, future prospects, and making it work for you. Ultrasound Q. 2011;27:177–86. doi: 10.1097/RUQ.0b013e31822a2138. [DOI] [PubMed] [Google Scholar]
- 4.Ophir J, Cespedes I, Ponnekanti H, et al. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13:111–34. doi: 10.1177/016173469101300201. [DOI] [PubMed] [Google Scholar]
- 5.Lerner RM, Huang SR, Parker KJ. “Sonoelasticity” images derived from ultrasound signals in mechanically vibrated tissues. Ultrasound Med Biol. 1990;16:231–39. doi: 10.1016/0301-5629(90)90002-t. [DOI] [PubMed] [Google Scholar]
- 6.Itoh A, Ueno E, Tohno E, et al. Breast disease: Clinical application of US elastography for diagnosis. Radiology. 2006;239:341–50. doi: 10.1148/radiol.2391041676. [DOI] [PubMed] [Google Scholar]
- 7.Tristam M, Barbosa DC, Cosgrove DO, et al. Ultrasonic study of in vivo kinetic characteristics of human tissues. Ultrasound Med Biol. 1986;12:927–37. doi: 10.1016/0301-5629(86)90061-x. [DOI] [PubMed] [Google Scholar]
- 8.Tristam M, Barbosa DC, Cosgrove DO, et al. Application of Fourier analysis to clinical study of patterns of tissue movement. Ultrasound Med Biol. 1988;14:695–707. doi: 10.1016/0301-5629(88)90026-9. [DOI] [PubMed] [Google Scholar]
- 9.Levinson SF, Shinagawa M, Sato T. Sonoelastic determination of human skeletal muscle elasticity. J Biomech. 1995;28:1145–54. doi: 10.1016/0021-9290(94)00173-2. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Snedeker JG. Elastography: modality-specific approaches, clinical applications, and research horizons. Skeletal Radiol. 2011;40:389–97. doi: 10.1007/s00256-010-0918-0. [DOI] [PubMed] [Google Scholar]
- 11.Lalitha P, Reddy MCh, Reddy KJ. Musculoskeletal applications of elastography: A pictorial essay of our initial experience. Korean J Radiol. 2011;12:365–75. doi: 10.3348/kjr.2011.12.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park GY, Kwon DR. Application of real-time sonoelastography in musculoskeletal diseases related to physical medicine and rehabilitation. Am J Phys Med Rehabil. 2011;90(11):875–86. doi: 10.1097/PHM.0b013e31821a6f8d. [DOI] [PubMed] [Google Scholar]
- 13.Drakonaki EE, Allen GM, Wilson DJ. Real-time ultrasound elastography of the normal Achilles tendon: reproducibility and pattern description. Clin Radiol. 2009;64:1196–202. doi: 10.1016/j.crad.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 14.De Zordo T, Chem R, Smekal V, et al. Real-time sonoelastography: Findings in patients with symptomatic achilles tendons and comparison to healthy volunteers. Ultraschall Med. 2010;31:394–400. doi: 10.1055/s-0028-1109809. [DOI] [PubMed] [Google Scholar]
- 15.De Zordo T, Fink C, Feuchtner GM, et al. Real-time sonoelastography findings in healthy Achilles tendons. Am J Roentgenol. 2009;193:W134–38. doi: 10.2214/AJR.08.1843. [DOI] [PubMed] [Google Scholar]
- 16.Klauser AS, Faschingbauer R, Jaschke WR. Is sonoelastography of value in assessing tendons? Semin Musculoskelet Radiol. 2010;14:323–33. doi: 10.1055/s-0030-1254521. [DOI] [PubMed] [Google Scholar]
- 17.De Sconfienza LM, Silvestri E, Cimmino MA. Sonoelastography in the evaluation of painful Achilles tendon in amateur athletes. Clin Exp Rheumatol. 2010;28:373–78. [PubMed] [Google Scholar]
- 18.De Zordo T, Lill SR, Fink C, et al. Real-time sonoelastography of lateral epicondylitis: comparison of findings between patients and healthy volunteers. Am J Roentgenol. 2009;193:180–85. doi: 10.2214/AJR.08.2020. [DOI] [PubMed] [Google Scholar]
- 19.Niitsu M, Michizaki A, Endo A, et al. Muscle hardness measurement by using ultrasound elastography: a feasibility study. Acta Radiol. 2011;52:99–105. doi: 10.1258/ar.2010.100190. [DOI] [PubMed] [Google Scholar]
- 20.Ariji Y, Katsumata A, Hiraiwa Y, et al. Use of sonographic elastography of the masseter muscles for optimizing massage pressure: a preliminary study. J Oral Rehabil. 2009;36:627–35. doi: 10.1111/j.1365-2842.2009.01977.x. [DOI] [PubMed] [Google Scholar]
- 21.Detorakis ET, Drakonaki EE, Tsilimbaris MK, et al. Real-time ultrasound elastographic imaging of ocular and periocular tissues: a feasibility study. Ophthalmic Surg Lasers Imaging. 2010;41:135–41. doi: 10.3928/15428877-20091230-24. [DOI] [PubMed] [Google Scholar]
- 22.Botar-Jid C, Damian L, Dudea SM, et al. The contribution of ultrasonography and sonoelastography in assessment of myositis. Med Ultrason. 2010;12:120–26. [PubMed] [Google Scholar]
- 23.Drakonaki EE, Allen GM. Magnetic resonance imaging, ultrasound and real-time ultrasound elastography of the thigh muscles in congenital muscle dystrophy. Skeletal Radiol. 2010;39:391–96. doi: 10.1007/s00256-009-0861-0. [DOI] [PubMed] [Google Scholar]
- 24.Sikdar S, Shah JP, Gebreab T, et al. Novel applications of ultrasound technology to visualize and characterize myofascial trigger points and surrounding soft tissue. Arch Phys Med Rehabil. 2009;90:1829–38. doi: 10.1016/j.apmr.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasilescu D, Vasilescu D, Dudea S, et al. Sonoelastography contribution in cerebral palsy spasticity treatment assessment, preliminary report: a systematic review of the literature apropos of seven patients. Med Ultrason. 2010;12:306–10. [PubMed] [Google Scholar]
- 26.Silvestri E, Garlaschi G, Bartolini B, et al. Sonoelastography can help in the localization of soft tissue damage in polymyalgia rheumatica (PMR) Clin Exp Rheumatol. 2007;25:796. [PubMed] [Google Scholar]
- 27.Cimmino MA, Grassi W. What is new in ultrasound and magnetic resonance imaging for musculoskeletal disorders? Best Pract Res Clin Rheumatol. 2008;22:1141–48. doi: 10.1016/j.berh.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Iagnocco A, Kaloudi O, Perella C, et al. Ultrasound elastography assessment of skin involvement in systemic sclerosis: lights and shadows. J Rheumatol. 2010;37:1688–91. doi: 10.3899/jrheum.090974. [DOI] [PubMed] [Google Scholar]
- 29.Sconfienza LM, Silvestri E, Bartolini B, et al. Sonoelastography may help in the differential diagnosis between rheumatoid nodules and tophi. Clin Exp Rheumatol. 2010;28:144–45. [PubMed] [Google Scholar]
- 30.Bhatia KS, Rasalkar DD, Lee YP, et al. Real-time qualitative ultrasound elastography of miscellaneous non-nodal neck masses: applications and limitations. Ultrasound Med Biol. 2010;36:1644–52. doi: 10.1016/j.ultrasmedbio.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Itoh A, Ueno E, Tohno E, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239:341–50. doi: 10.1148/radiol.2391041676. [DOI] [PubMed] [Google Scholar]
- 32.Pallwein L, Mitterberger M, Struve P, et al. Real-time elastography for detecting prostate cancer: preliminary experience. BJU Int. 2007;100:42–46. doi: 10.1111/j.1464-410X.2007.06851.x. [DOI] [PubMed] [Google Scholar]
- 33.Dighe M, Bae U, Richardson ML, et al. Differential diagnosis of thyroid nodules with US elastography using carotid artery pulsation. Radiology. 2008;248:662–69. doi: 10.1148/radiol.2482071758. [DOI] [PubMed] [Google Scholar]
- 34.Thomas A, Kümmel S, Gemeinhardt O, Fischer T. Real-time sonoelastography of the cervix: tissue elasticity of the normal and abnormal cervix. Acad Radiol. 2007;14:193–200. doi: 10.1016/j.acra.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Saftoiu A, Vilmann P, Hassan H, Gorunescu F. Analysis of endoscopic ultrasound elastography used for characterisation and differentiation of benign and malignant lymph nodes. Ultraschall Med. 2006;27:535–42. doi: 10.1055/s-2006-927117. [DOI] [PubMed] [Google Scholar]
- 36.Janssen J, Schlorer E, Greiner L. EUS elastography of the pancreas: feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointest Endosc. 2007;65:971–78. doi: 10.1016/j.gie.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 37.Friedrich-Rust M, Ong MF, Herrmann E, et al. Real-time elastography for noninvasive assessment of liver fibrosis in chronic viral hepatitis. Am J Roentgenol. 2007;188:758–64. doi: 10.2214/AJR.06.0322. [DOI] [PubMed] [Google Scholar]
- 38.Goertz RS, Zopf Y, Jugl V, et al. Measurement of liver elasticity with acoustic radiation force impulse (ARFI) technology: An alternative noninvasive method for staging liver fibrosis in viral hepatitis. Ultraschall Med. 2010;31:151–55. doi: 10.1055/s-0029-1245244. [DOI] [PubMed] [Google Scholar]
- 39.Tozaki M, Isobe S, Fukuma E. Preliminary study of ultrasonographic tissue quantification of the breast using the acoustic radiation force impulse (ARFI) technology. Eur J Radiol. 2011;80:e182–87. doi: 10.1016/j.ejrad.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 40.Friedrich-Rust M, Romenski O, Meyer G, et al. Acoustic Radiation Force Impulse-Imaging for the evaluation of the thyroid gland: A limited patient feasibility study. Ultrasonics. 2012;52:69–74. doi: 10.1016/j.ultras.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Drakonaki E, Allen GM, Wilson DJ. Ultrasound elastography for musculoskeletal applications. Br J Radiol. 2012;85(1019):1435–45. doi: 10.1259/bjr/93042867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bercoff J, Tanter M, Fink M. Supersonic shear imaging: A new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:396–409. doi: 10.1109/tuffc.2004.1295425. [DOI] [PubMed] [Google Scholar]
- 43.Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–13. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Gennisson JL, Deffieux T, Macé E, et al. Viscoelastic and anisotropic mechanical properties of in vivo muscle tissue assessed by supersonic shear imaging. Ultrasound Med Biol. 2010;36:789–801. doi: 10.1016/j.ultrasmedbio.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 45.Nordez A, Gennisson JL, Casari P, et al. Characterization of muscle belly elastic properties during passive stretching using transient elastography. J Biomech. 2008;41:2305–11. doi: 10.1016/j.jbiomech.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 46.Sabra KG, Conti S, Roux P, Kuperman WA. Passive in vivo elastography from skeletal muscle noise. Appl Phys Lett. 2007;90:194101–3. [Google Scholar]
- 48.Grainger AJ. Highlights of the European Society of Musculoskeletal Radiology (ESSR) annual meeting 2010. Skeletal Radiol. 2011;40:137–39. doi: 10.1007/s00256-010-1030-1. [DOI] [PubMed] [Google Scholar]
- 47.Arda K, Ciledag N, Aktas E, et al. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. Am J Roentgenol. 2011;197:532–36. doi: 10.2214/AJR.10.5449. [DOI] [PubMed] [Google Scholar]
- 49.Schreiber V, Smekal V, De Zordo T, et al. Real-time sonoelastography in rotator cuff imaging and comparison to magnetic resonance imaging as gold standard. RSNA. 2009 Dec 1; Available. [Google Scholar]
- 50.Clinical abstracts. Tokyo, Japan: Hitachi; Hitachi Hitachi real-time tissue elastography: publications & international communications. Available from: http://www.hitachi-medical-systems.eu/fileadmin/hitachi_en/downloads/hi-rte-publications-and-communications-clinical-abstracts---musculoskeletal-applications-11-06-10.pdf. [Google Scholar]
- 51.Geier B, Barbera L, Muth-Werthmann D, et al. Ultrasound elastography for the age determination of venous thrombi – Evaluation in an animal model of venous thrombosis. Thromb Haemost. 2005;93:368–74. doi: 10.1160/TH04-07-0437. [DOI] [PubMed] [Google Scholar]


