Abstract
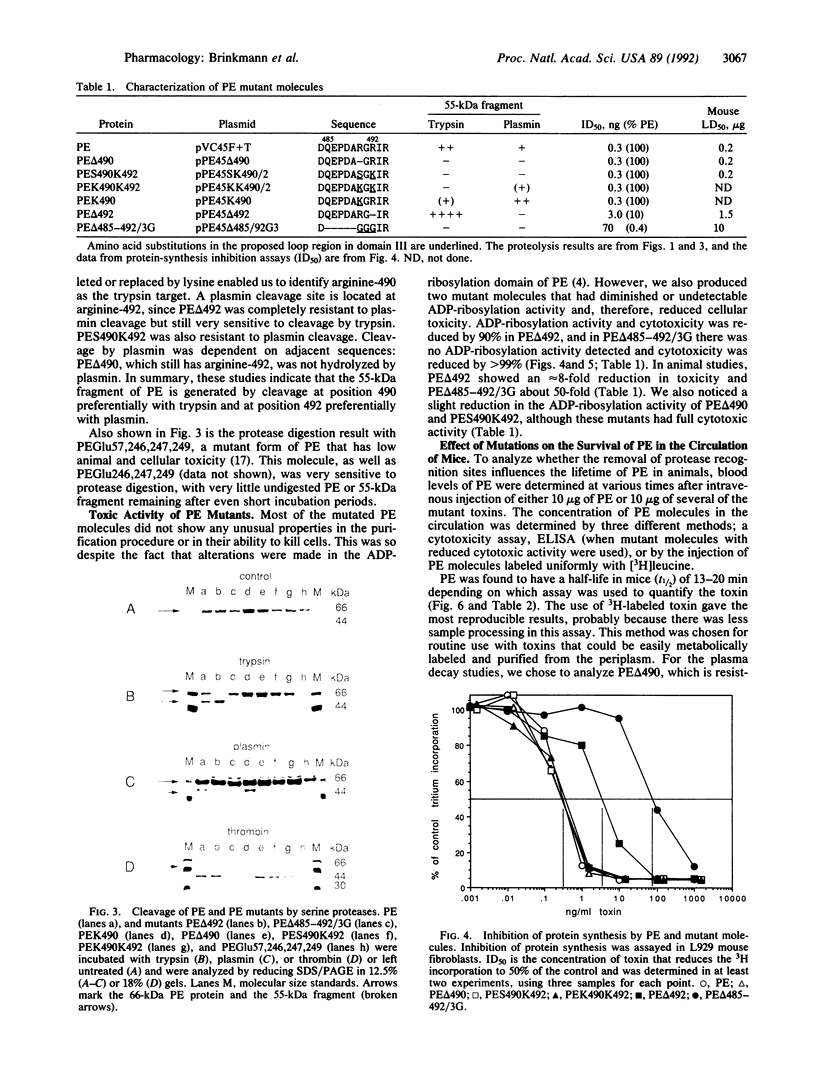
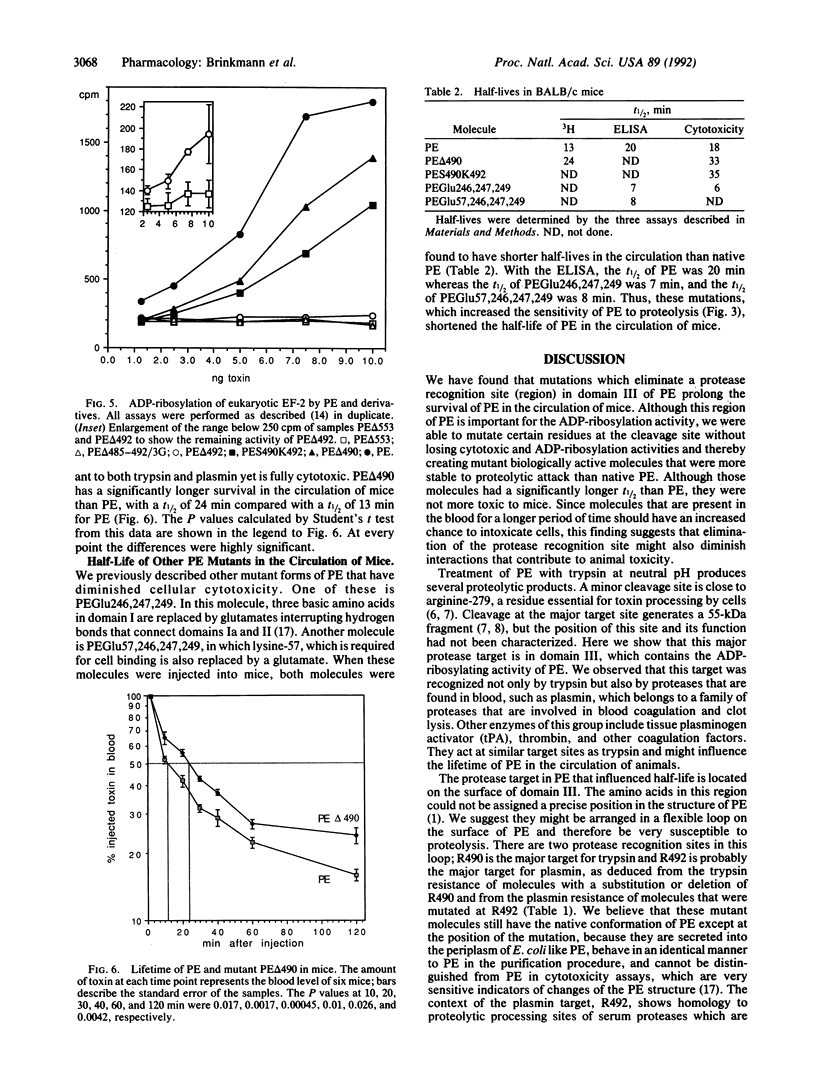
Pseudomonas exotoxin A (PE) is a single-chain 66-kDa polypeptide that kills eukaryotic cells by ADP-ribosylation of translational elongation factor 2. PE is composed of three major structural domains whose functions are binding of cells (I), translocation (II), and ADP-ribosylation (III). Here we describe a protease cleavage target that is located near arginine-490 on the surface of domain III. We made several different types of mutations near arginine-490. Deletion of arginine-490 or replacement of arginine-490 and -492 with serine and lysine or with two lysines resulted in protease-resistant molecules that were fully cytotoxic and had normal ADP-ribosylation activity. However, the half-life in mouse blood of the PE delta 490 mutant was 24 min whereas that of PE was 13 min. Furthermore, two PE mutants that were protease-hypersensitive, PEGlu246,247,249 and PEGlu57,246,247,249 (in which glutamate residues replace basic residues at the indicated positions), had very short half-lives. These data indicate that protease sensitivity is an important determinant in the half-life of PE in the circulation and suggest that the half-life of other proteins may be prolonged by removal of protease sites. Deletion of arginine-492 or the replacement of amino acids 486-491 with three glycines markedly diminished ADP-ribosylation activity and cytotoxicity, indicating that this region of domain III is also important for catalytic activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allured V. S., Collier R. J., Carroll S. F., McKay D. B. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0-Angstrom resolution. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1320–1324. doi: 10.1073/pnas.83.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer P. I., Machovich R., Büki K. G., Csonka E., Koch S. A., Horváth I. Interaction of plasmin with endothelial cells. Biochem J. 1984 Feb 15;218(1):119–124. doi: 10.1042/bj2180119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary V. K., FitzGerald D. J., Adhya S., Pastan I. Activity of a recombinant fusion protein between transforming growth factor type alpha and Pseudomonas toxin. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4538–4542. doi: 10.1073/pnas.84.13.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary V. K., Jinno Y., Gallo M. G., FitzGerald D., Pastan I. Mutagenesis of Pseudomonas exotoxin in identification of sequences responsible for the animal toxicity. J Biol Chem. 1990 Sep 25;265(27):16306–16310. [PubMed] [Google Scholar]
- Douglas C. M., Collier R. J. Exotoxin A of Pseudomonas aeruginosa: substitution of glutamic acid 553 with aspartic acid drastically reduces toxicity and enzymatic activity. J Bacteriol. 1987 Nov;169(11):4967–4971. doi: 10.1128/jb.169.11.4967-4971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald D., Pastan I. Targeted toxin therapy for the treatment of cancer. J Natl Cancer Inst. 1989 Oct 4;81(19):1455–1463. doi: 10.1093/jnci/81.19.1455. [DOI] [PubMed] [Google Scholar]
- Forsgren M., Råden B., Israelsson M., Larsson K., Hedén L. O. Molecular cloning and characterization of a full-length cDNA clone for human plasminogen. FEBS Lett. 1987 Mar 23;213(2):254–260. doi: 10.1016/0014-5793(87)81501-6. [DOI] [PubMed] [Google Scholar]
- Gray G. L., Smith D. H., Baldridge J. S., Harkins R. N., Vasil M. L., Chen E. Y., Heyneker H. L. Cloning, nucleotide sequence, and expression in Escherichia coli of the exotoxin A structural gene of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1984 May;81(9):2645–2649. doi: 10.1073/pnas.81.9.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J., Fitzgerald D. J., Adhya S., Pastan I. Functional domains of Pseudomonas exotoxin identified by deletion analysis of the gene expressed in E. coli. Cell. 1987 Jan 16;48(1):129–136. doi: 10.1016/0092-8674(87)90363-1. [DOI] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J. X., London E. Involvement of denaturation-like changes in Pseudomonas exotoxin a hydrophobicity and membrane penetration determined by characterization of pH and thermal transitions. Roles of two distinct conformationally altered states. J Biol Chem. 1990 May 25;265(15):8636–8641. [PubMed] [Google Scholar]
- Jinno Y., Chaudhary V. K., Kondo T., Adhya S., FitzGerald D. J., Pastan I. Mutational analysis of domain I of Pseudomonas exotoxin. Mutations in domain I of Pseudomonas exotoxin which reduce cell binding and animal toxicity. J Biol Chem. 1988 Sep 15;263(26):13203–13207. [PubMed] [Google Scholar]
- Jinno Y., Ogata M., Chaudhary V. K., Willingham M. C., Adhya S., FitzGerald D., Pastan I. Domain II mutants of Pseudomonas exotoxin deficient in translocation. J Biol Chem. 1989 Sep 25;264(27):15953–15959. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ogata M., Chaudhary V. K., Pastan I., FitzGerald D. J. Processing of Pseudomonas exotoxin by a cellular protease results in the generation of a 37,000-Da toxin fragment that is translocated to the cytosol. J Biol Chem. 1990 Nov 25;265(33):20678–20685. [PubMed] [Google Scholar]
- Pastan I., FitzGerald D. Recombinant toxins for cancer treatment. Science. 1991 Nov 22;254(5035):1173–1177. doi: 10.1126/science.1683495. [DOI] [PubMed] [Google Scholar]
- Pastan I., Willingham M. C., FitzGerald D. J. Immunotoxins. Cell. 1986 Dec 5;47(5):641–648. doi: 10.1016/0092-8674(86)90506-4. [DOI] [PubMed] [Google Scholar]
- Prior T. I., FitzGerald D. J., Pastan I. Barnase toxin: a new chimeric toxin composed of pseudomonas exotoxin A and barnase. Cell. 1991 Mar 8;64(5):1017–1023. doi: 10.1016/0092-8674(91)90325-s. [DOI] [PubMed] [Google Scholar]
- Spolarics Z., Kalapos M. P., Léránt I., Garzó T., Antoni F., Mandl J., Machovich R. Association of thrombin, plasmin, thrombin-antithrombin III complex and plasmin-antithrombin III complex with isolated hepatocytes. Biochim Biophys Acta. 1989 Aug 15;1012(3):231–236. doi: 10.1016/0167-4889(89)90102-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- van Zonneveld A. J., Veerman H., MacDonald M. E., van Mourik J. A., Pannekoek H. Structure and function of human tissue-type plasminogen activator (t-PA). J Cell Biochem. 1986;32(3):169–178. doi: 10.1002/jcb.240320302. [DOI] [PubMed] [Google Scholar]