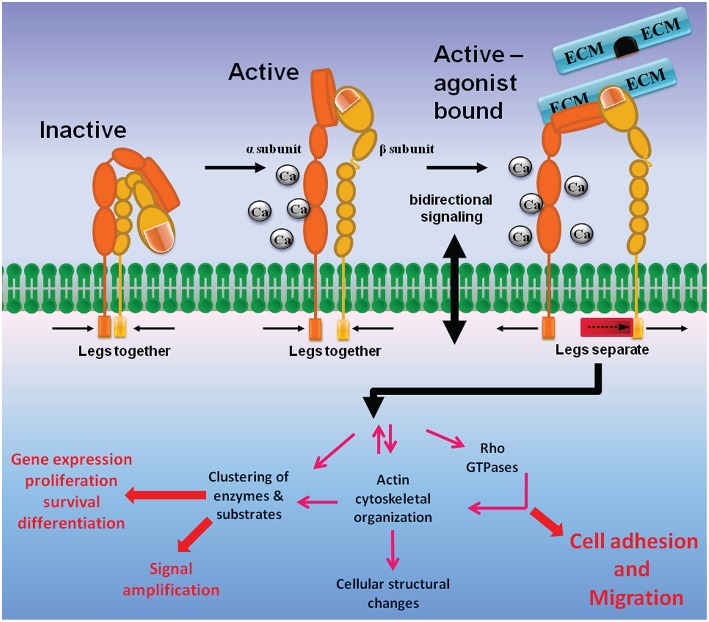
Figure 1.
Integrin structure and bidirectional signaling. Integrins are membrane-spanning glycoprotein heterodimers that mediate cell adhesion and migration. The large extracellular domains contain sites for ligand and cation binding, while the small intracellular region senses changes in intracellular signaling pathways that convey information to the ECM via conformational modifications. Inactive integrins are present in a bent conformation (low affinity, left). Intracellular activation signals induce integrins to attain an upright position with a dual extended head-piece arrangement (middle). The upright conformation is characterized by an intermediate affinity state and a high affinity, ligand-bound state where the integrin α and β subunit tails are separated. Integrin inside-out signaling is achieved when intracellular signaling proteins push the β integrin leg away from the α integrin subunit, allowing for formation of an integrin high affinity activation state (right). Stabilization of integrin binding to ECM initiates outside-in signaling, where the integrins mediate downstream signaling that includes focal adhesion and actin stress fiber formations, activation of Rho GTPases, and cellular structural changes, etc., which ultimately leads to promotion of cell adhesion and migration.