Abstract
The homeodomain-leucine zipper (HD-Zip) transcription factor family is a key transcription factor family and unique to the plant kingdom. It consists of a homeodomain and a leucine zipper that serve in combination as a dimerization motif. The family can be classified into four subfamilies, and these subfamilies participate in the development of hormones and mediation of hormone action and are involved in plant responses to environmental conditions. However, limited information on this gene family is available for the important chrysanthemum ornamental species (Chrysanthemum morifolium). Here, we characterized 17 chrysanthemum HD-Zip genes based on transcriptome sequences. Phylogenetic analyses revealed that 17 CmHB genes were distributed in the HD-Zip subfamilies I and II and identified two pairs of putative orthologous proteins in Arabidopsis and chrysanthemum and four pairs of paralogous proteins in chrysanthemum. The software MEME was used to identify 7 putative motifs with E values less than 1e-3 in the chrysanthemum HD-Zip factors, and they can be clearly classified into two groups based on the composition of the motifs. A bioinformatics analysis predicted that 8 CmHB genes could be targeted by 10 miRNA families, and the expression of these 17 genes in response to phytohormone treatments and abiotic stresses was characterized. The results presented here will promote research on the various functions of the HD-Zip gene family members in plant hormones and stress responses.
Keywords: Chrysanthemum morifolium, HD-Zip, phylogenetic analysis, stress response
1. Introduction
Transcription factors (TFs) play crucial roles in plant development, growth, and responses to various environmental conditions. The homeodomain-leucine zipper (HD-Zip) transcription factor family is one of the key transcription factor families [1], and it is unique to the plant kingdom, although it is found in the recently identified charophycean algae [2]. Members of the HD-Zip family contain a special conserved HD domain that is responsible for its specific binding to DNA and an adjacent leucine zipper motif (LZ) that is a dimerization motif. The homeodomain is a 60–61 amino acid DNA-binding domain composed of three alpha helices, wherein the second and third helices form a helix-turn-helix DNA-binding motif capable of interacting specifically with DNA [3]. The HD-Zip proteins bind to DNA as dimers, and the LZ motif promotes the formation of homo- and hetero-dimers for the efficient recognition of DNA.
The HD-Zip family can be classified into four subfamilies, HD-Zip I to IV, based on four distinguishing characteristics: HD-Zip domain conservation, additional conserved domains, gene structures and physiological functions [4]. The HD-Zip I proteins exhibit a highly conserved HD, a less conserved LZ and no other similarities. The HD-Zip II subfamily contains two additional motifs: the CPSCE (named after the five conserved amino acids Cys, Pro, Ser, Cys, Glu in the one letter code) and an N-terminal consensus sequence. Compared with subfamily I, subfamily III shows additional amino acids in the binding domain between the HD and the LZ motifs; this binding domain includes a conserved START (steroidogenic acute regulatory protein-related lipid transfer) domain, an adjacent conserved SAD (START-adjacent domain) region, and a MEKHLA domain in the C-terminus. Subfamily IV proteins are HD-Zip-START-SAD and similar to those of subfamily III, although the most distinguishable features are the presence of a loop in the middle of the LZ domain and the lack of an additional C-terminal MEKHLA motif. The proteins encoded by HD-Zip I form dimers that recognize the pseudo palindromic sequence CAAT(A/T)ATTG [5]. The HD-Zip II proteins bind to the same sequence and differ from the members of the HD-Zip I family only in the recognized nucleotide located in the center of the target site [6]. HD-Zip III proteins can interact with GTAAT(G/C)ATTAC in vitro [7], whereas HD-Zip IV proteins show a binding preference for the alternative sequence TAAATG(C/T)A [8].
Many members of the HD-Zip protein have been analyzed in various plant species, including Arabidopsis (Arabidopsis thaliana) [9], rice (Oryza sativa) [10], maize (Zea mays) [11], tobacco (Nicotiana sylvestris) [12], tomato (Solanum lycopersicum) [13], barley (Hordeum vulgare) [14], soybean (Glycine max) [15], Medicago truncatula [16], sunflower (Heliantus annuus) [17], moss (Physcomitrella patens) [18], and poplar (Populus trichocarpa) [19]. The structural and functional characterization of HD-Zip proteins was limited to Arabidopsis when only a few HD-Zip proteins had been identified in other species. Currently, there are 47 HD-Zip transcription factors known in A. thaliana, including 17 members of HD-Zip I, 9 members of HD-Zip II, 5 members of HD-Zip III, and 16 members of HD-Zip IV, and they play various roles in plant development.
The expression of HD-Zip I proteins is regulated by abiotic stresses, including drought, extreme temperatures, osmotic stress and illumination conditions, and the proteins are involved in responses to these environmental conditions as well as de-etiolation [20]. For example, ATHB1, the first member of HD-Zip, functions as a mediator in the determination of leaf cell fate [21]. Zhao et al. found 17 HD-Zip transcription factors in maize that were regulated by drought stress [11]. A novel maize HD-Zip I gene, Zmhdz10, which is induced by drought, salt stress and abscisic acid (ABA), can positively regulate drought and salt tolerance in plants through an ABA-dependent signaling pathway [22]. ATHB5 might contribute to the spatial regulation of BDL expression to modulate the BDL-dependent auxin response [23]. An in situ hybridization analysis of Vrs1 from barley indicated that it encodes an HD-Zip I transcription factor and is expressed exclusively in the pistil, lemma, palea and lodicule of the lateral spikelets; moreover, Vrs1 was also found to inhibit gynoecial development, thus showing the neofunctionalization of the HD-Zip I subfamily [24].
HD-Zip II proteins are mainly involved in plant responses to illumination conditions, shade avoidance and auxin signaling [25]. ATHB2 can be induced rapidly by changes in the red to far-red ratio to promote the shade-avoidance response in the majority of angiosperms [9], whereas HAT2 is involved in the regulation of auxin-mediated morphogenesis in the shoot and root tissues [26]. Furthermore, recent studies have revealed additional functions of this subfamily in plant development, including carpel margin development [27] and leaf polarity [28]. The progressive loss of activity of HOMEOBOX 2 (ATHB2), HAT3 and ATHB4 (HD-Zip II proteins in Arabidopsis) in A. thaliana causes developmental defects during embryogenesis, suggesting that these regulators control apical embryo development and meristem regulation [29].
HD-Zip III proteins are regulators of apical meristem, embryogenesis, auxin transport, leaf polarity, later organ initiation and vascular system development; thus, they play overlapping, antagonistic or distinct roles [30,31]. Plants that overexpress ATHB8 show an overproduction of xylem, indicating that this gene plays a crucial role in vascular development [32]. PtaHB1 overexpression produces noticeable effects on petiole and primary shoot fiber development, suggesting that PtaHB1 is involved in secondary vascular growth in angiosperms and gymnosperms [31]. The popREVOLUTA (PRE) gene from Populus plays a fundamental role in the initiation of the cambium and the regulation of secondary vascular tissue patterning [33].
HD-Zip IV proteins play crucial roles in epidermal cell differentiation, anthocyanin accumulation, root development, trichome formation and cuticle development [34]. The HD-Zip IV gene HDG11 can improve drought tolerance and increase grain yield in transgenic rice plants [35]. ATML1 regulates gene expression in the epidermis specification [8], PROTODERMAL FACTOR2 (PDF2) is crucial for normal development of floral organs in Arabidopsis [36]. Moreover, ATML1 and PDF2 act redundantly as a positive regulator of shoot epidermal cell differentiation and at least one copy of these genes is essential for embryo development [37]. GLABRA2 (GL2) is required for the differentiation of epidermal cells in Arabidopsis tricomes, and it activates a positive feedback loop via MYB23 [38].
HD-Zip genes are also involved in regulating the adaptive response of plants to biotic stresses, including the microbes Pseudomonas syringae [39] and Alternaria alternate [40] and the insects Spodoptera littoralis and S. frugiperda [41].
Recently, a genome-wide and expression analysis of the HD-Zip transcription factors was performed for various species, including peach with 33 members [42], pear with 52 members [40] and soybean with 101 members [43]. The RNA-seq approach indicated that five HD-Zip I genes and one HD-Zip II gene showed differential expression under dehydration stress, while seven HD-Zip I, four HD-Zip II, oneHD-Zip III, and four HD-Zip IV genes showed differential expression under salt stress in soybean [43]. However, the systematic identification of sequences and expression patterns under abiotic stress has not been conducted for chrysanthemum. Chrysanthemum (Chrysanthemum morifolium) is one of the four most famous cut flowers in the world, and a perennial Asteraceae species; however, it is susceptible to various biotic and abiotic stresses [44]. Asteraceae is one of the largest families of flowering plants with more than 23,000 species, but rare information was available about HD-Zip gene family. For this study, we isolated 17 HD-Zip genes in chrysanthemum based on a set of transcriptome data. We performed a comparative phylogenetic analysis of chrysanthemum and Arabidopsis genes in silico and investigated the transcript levels in response to various phytohormones and abiotic stresses using qRT-PCR. The results provided novel insights into the stress responses of CmHB genes and provided a better understanding of the structure and function of the HD-Zip factors in chrysanthemum plants.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
Cuttings of the cut-flower chrysanthemum cultivar “Jinba”, which is maintained at the Chrysanthemum Germplasm Resource Preservation Center (Nanjing Agricultural University, Nanjing, China), were rooted in vermiculite plus plain water without fertilizer in a greenhouse. After 14 days, the plants were transplanted to a growth substrate (1:1 mixture of garden soil and vermiculite) and subjected to a range of stress and phytohormone treatments.
2.2. Plant Treatments
Tissue-specific and treatment-induced transcription profiles of 17 CmHB genes were explored in the roots, stems and leaves of young seedlings as well as in the tube and ray florets of inflorescences at the bud stage. A variety of abiotic stresses were imposed, including high salinity (200 mM NaCl) and drought (20% w/v polyethylene glycol (PEG6000)) [45].
For the NaCl and PEG6000 stresses, the six-to-eight-leaf stage plants were transferred to liquid medium containing the stress agent, and the second true leaves were sampled at various times [46]. The wounding treatment involved cutting the second true leaf, and the phytohormone treatments involved spraying the leaves with 50 μM ABA, 1 mM methyl jasmonate (MeJA) or 200 μM salicylic acid (SA) [47]. The plants were sampled prior to the treatment and then at 1, 4, 12 and 24 h after the treatment.
After sampling, all of the collected material was snap frozen in liquid nitrogen and stored at −70 °C. Each treatment was replicated three times.
2.3. Transcriptome Search and Sequencing of Full-Length CmHB cDNAs
All of the putative HD-Zip proteins were retrieved from C. morifolium transcriptome data [48]. Arabidopsis HD-Zip protein sequences were downloaded from The Arabidopsis Information Resource (TAIR) database. The chrysanthemum transcriptome was searched to identify HD-Zip proteins using Basic Local Alignment Search Tool algorithms (tBLASTx) and the published Arabidopsis HD-Zip protein sequences as query sequences. All of the obtained protein sequences were examined for the presence of the HD and LZ domains using the Pfam (http://pfam.sanger.ac.uk/search) and SMART (http://smart.embl-heidelberg.de/) tools. Multiple alignments among the identified CmHB sequences were also performed to avoid repetition. Furthermore, the full open reading frames of the CmHB sequences were obtained via RACE PCR. First-strand cDNA was synthesized using the dT adaptor primer dT-AP and then subjected to nested PCR using the primer pair CmHBx-3-F1/F2 and the adaptor primer AP (Table S1). Finally, 17 pairs of gene-specific primers (Table S2) were designed to amplify complete open reading frames. The amplicons were purified using AxyPrep DNA Gel Extraction Kits (Axygen, Hangzhou, China) and cloned into pMD19-T (TaKaRa, Tokyo, Japan) for sequencing.
2.4. Phylogenetic Tree Construction and Sequence Analysis
A phylogenetic tree was constructed with MEGA version 6.0 using the maximum likelihood method [49]. ClustalW software was employed for multi-sequence alignments of HD-Zip I & II TFs between Arabidopsis and C. morifolium [50]. Internal branching support was estimated using 500 bootstrap replicates. The theoretical isoelectric point (pI) and molecular weight (Mw) of the CmHB proteins were calculated using the Compute pI/Mw online tool (http://web.expasy.org/compute_pi/), and subcellular localization was predicted with PSORT [51]. Putative conserved motifs were predicted using the MEME program v4.10.2 [52] with the following parameters: any number of repetitions; motif sites, at least 3 sites; optimum motif widths between 6 and 61 residues; and E-value less than 1e-3. All of the motifs identified by MEME were searched in the InterPro database using InterProScan [53]. The target prediction for miRNA was performed using the psRNATarget online tool [54].
2.5. Real-Time Quantitative PCR (qPCR)
Total RNA was isolated from the samples using the RNAiso reagent (TaKaRa) according to the manufacturer’s instructions. The RNA was then treated with RNase-free DNase I (TaKaRa) to remove potential genomic DNA contamination. First-strand cDNA was synthesized from 1 μg of total RNA using SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The qPCR was performed using a Mastercycler ep realplex instrument (Eppendorf, Hamburg, Germany). Each 20 μL amplification reaction contained 10 μL of SYBR® Premix Ex Taq™ II (TaKaRa), 0.4 μL of each primer (10 μM), 4.2 μL of H2O and 5 μL of cDNA template. The PCR cycling regime consisted of an initial denaturation (95 °C for 2 min) followed by 40 cycles of 95 °C for 10 s, 55 °C for 15 s and 72 °C for 20 s. A melting curve analysis was performed following each assay to confirm the specificity of and efficiency of the primer pairs. Gene-specific primers (provided in Table S3) were designed using Primer3 Release 2.3.4 [55], and the EF1α gene was employed as a reference sequence [47]. The relative transcript abundance was calculated by the 2−ΔΔCT method [56]. Three independent experiments were performed.
2.6. Data Analysis
The relative expression levels of each CmHB gene were log2 transformed. The profiles were compared using Cluster v3.0 software [57] and visualized using Treeview [58]. SPSS v17.0 software (SPSS Inc., Chicago, IL, USA) was utilized for all statistical analyses.
3. Results
3.1. Identification and Phylogenetic Analysis of Putative HD-Zip Factors in Chrysanthemum
Seventeen chrysanthemum HD-Zip gene sequences were isolated and designated CmHB1 through CmHB17 (GenBank: KT253049–KT253065). The full-length cDNAs varied in length from 708 to 1263 bp, and the predicted protein products ranged from 166 (CmHB11) to 337 (CmHB17) amino acids. Details regarding the CmHB sequences are given in Table 1. All of the CmHB proteins are predicted to be localized in the nucleus.
Table 1.
Summary of CmHB sequences and the most similar A. thaliana homologs.
| Gene | GenBank Accession No. | Amino Acid Length (aa) | PI | MW | Subcellular Localization | Most Similar A. thaliana Homolog | Locus Name |
|---|---|---|---|---|---|---|---|
| CmHB1 | KT253049 | 211 | 4.98 | 24656.44 | N(9.05) | ATHB7 | AT2G46680.1 |
| CmHB2 | KT253050 | 301 | 8.82 | 33945.17 | N(9.30) | ATHB2 | AT4G16780.1 |
| CmHB3 | KT253051 | 292 | 4.85 | 34011.51 | N(7.93) | ATHB1 | AT3G01470.1 |
| CmHB4 | KT253052 | 318 | 8.7 | 35070.59 | N(9.40) | HAT3 | AT3G60390.1 |
| CmHB5 | KT253053 | 299 | 4.71 | 33985.07 | N(7.97) | ATHB1 | AT3G01470.1 |
| CmHB6 | KT253054 | 278 | 4.66 | 31919.69 | N(8.94) | ATHB1 | AT3G01470.1 |
| CmHB7 | KT253055 | 202 | 6.61 | 23500.1 | N(8.95) | ATHB7 | AT2G46680.1 |
| CmHB8 | KT253056 | 248 | 8.6 | 27814.11 | N(9.47) | HAT22 | AT4G37790.1 |
| CmHB9 | KT253057 | 273 | 8.46 | 30384.22 | N(9.32) | HAT22 | AT4G37790.1 |
| CmHB10 | KT253058 | 264 | 9.13 | 29681.66 | N(9.36) | HAT9 | AT2G22800.1 |
| CmHB11 | KT253059 | 166 | 8.85 | 19298.5 | N(8.76) | ATHB52 | AT5G53980.1 |
| CmHB12 | KT253060 | 237 | 5.45 | 27259.88 | N(8.66) | ATHB12 | AT3G61890.1 |
| CmHB13 | KT253061 | 311 | 5.91 | 35462.34 | N(9.18) | ATHB13 | AT1G69780.1 |
| CmHB14 | KT253062 | 292 | 5.76 | 33627.57 | N(8.85) | ATHB13 | AT1G69780.1 |
| CmHB15 | KT253063 | 275 | 4.69 | 31595.82 | N(8.68) | ATHB6 | AT2G22430.1 |
| CmHB16 | KT253064 | 297 | 4.91 | 34061.3 | N(7.97) | ATHB1 | AT3G01470.1 |
| CmHB17 | KT253065 | 337 | 7.63 | 37486.68 | N(9.08) | HAT14 | AT5G06710.1 |
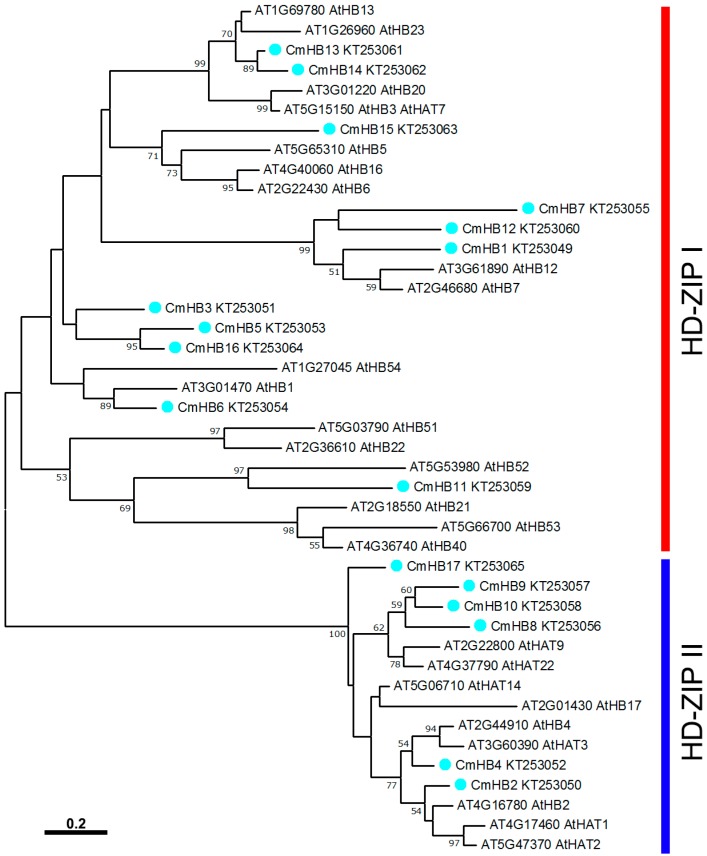
To evaluate the evolutionary relationships between the Arabidopsis and chrysanthemum HD-Zip I and II subfamily proteins, the deduced amino acid sequences of the identified HD-Zip genes were completely aligned. A combined phylogenetic tree (Figure 1) was then constructed using the Maximum Likelihood method and bootstrap analysis (500 reiterations), and the results showed diversification within the plant family (Figure 1). The seventeen CmHB genes were found to be distributed in the HD-Zip subfamilies I and II. Furthermore, two pairs of orthologous proteins were identified in Arabidopsis and chrysanthemum (AtHB1 with CmHB6 and AtHB52 with CmHB11), and four pairs of paralogous HD-Zip family proteins were identified in chrysanthemum (CmHB7 with CmHB12, CmHB9 with CmHB10, CmHB13 with CmHB14 and CmHB5 with CmHB16).
Figure 1.
Phylogenetic tree for the Arabidopsis and chrysanthemum HD-Zip I & II proteins. The tree was constructed from a complete alignment of 26 Arabidopsis and 17 chrysanthemum HD-Zip proteins using the Maximum Likelihood method. Bootstrap values below 50% have been omitted.
3.2. Conserved Sequences in Chrysanthemum HD-Zip Proteins
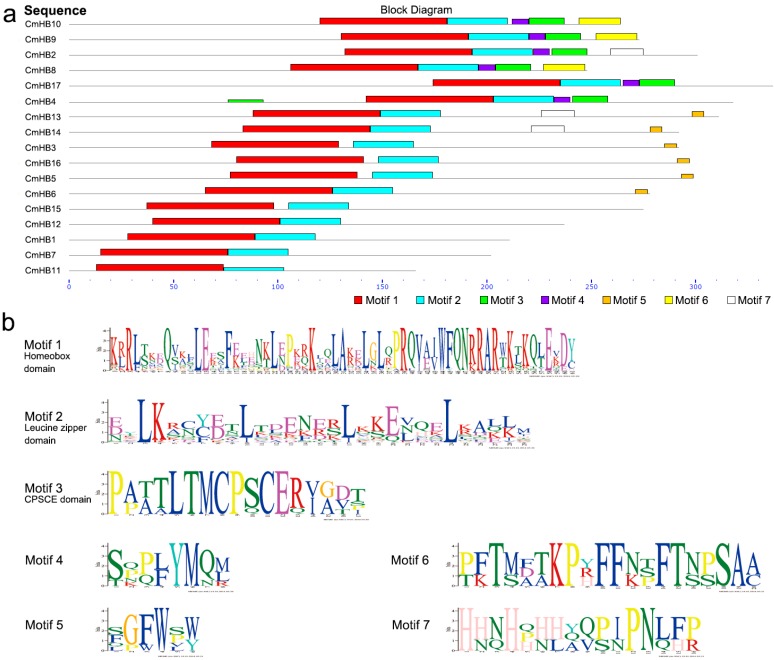
The software MEME was used to predict the motif composition of the HD-Zip factors, and 7 putative motifs with E values less than 1e-3 were identified (Figure 2). The HD-Zip TFs of the chrysanthemum can be clearly classified into two groups based on the motif composition (Figure 2a). For example, Motif 5 is only shared among the HD-Zip I subfamily members, whereas Motifs 3, 4, and 6 are shared among the HD-Zip II subfamily members. All of the chrysanthemum HD-Zip proteins have Motif 1 and Motif 2. Details of these motif features are shown in Figure 2b. The conserved sequences of these motifs were searched against the InterPro database. Motif 1 matched a homeobox domain, Motif 2 matched a leucine zipper domain, and Motif 3 matched a CPSCE domain; however, significant matches were not retrieved for the other motifs.
Figure 2.
Chrysanthemum HD-Zip protein motifs derived from the MEME analysis. (a) Distribution of conserved motifs in HD-Zip proteins; (b) sequences of motifs of HD-Zip proteins.
3.3. miRNA Target Site Prediction
All of the available plant miRNA data were used to predict candidates targeting CmHB transcripts. As shown in Table 2, 8 CmHBs are predicted to be targeted by 10 miRNA families. CmHB9 contains three target sites, CmHB4 contains two target sites, and the remaining 6 CmHBs contain only one target site. Additionally, miR414 can target CmHB4 and CmHB9.
Table 2.
The target prediction of miRNA.
| miRNA | Target | Exp | UPE | miRNA Start | miRNA End | Target Start | Target End |
|---|---|---|---|---|---|---|---|
| miR414 | CmHB4 | 2.5 | 20.266 | 1 | 21 | 512 | 532 |
| miR414 | CmHB9 | 2.5 | 10.125 | 1 | 21 | 460 | 480 |
| miR917 | CmHB9 | 2.5 | 18.596 | 1 | 20 | 360 | 379 |
| miR1023a-3p | CmHB13 | 3 | 13.468 | 1 | 20 | 135 | 154 |
| miR444 | CmHB16 | 2 | 9.773 | 1 | 21 | 537 | 557 |
| miR158a-5p | CmHB5 | 3 | 19.317 | 1 | 19 | 738 | 757 |
| miR4369 | CmHB17 | 3 | 16.17 | 1 | 20 | 368 | 387 |
| miR4993 | CmHB4 | 3 | 17.178 | 1 | 21 | 922 | 942 |
| miR5298 | CmHB10 | 3 | 5.976 | 1 | 22 | 135 | 156 |
| miR5380 | CmHB9 | 3 | 1.732 | 1 | 23 | 9 | 31 |
| miR5751 | CmHB14 | 3 | 16.3 | 1 | 20 | 134 | 153 |
Exp: Expectation; UPE: allowed maximum energy to unpair the target site.
3.4. Transcription Profiling of CmHB Genes
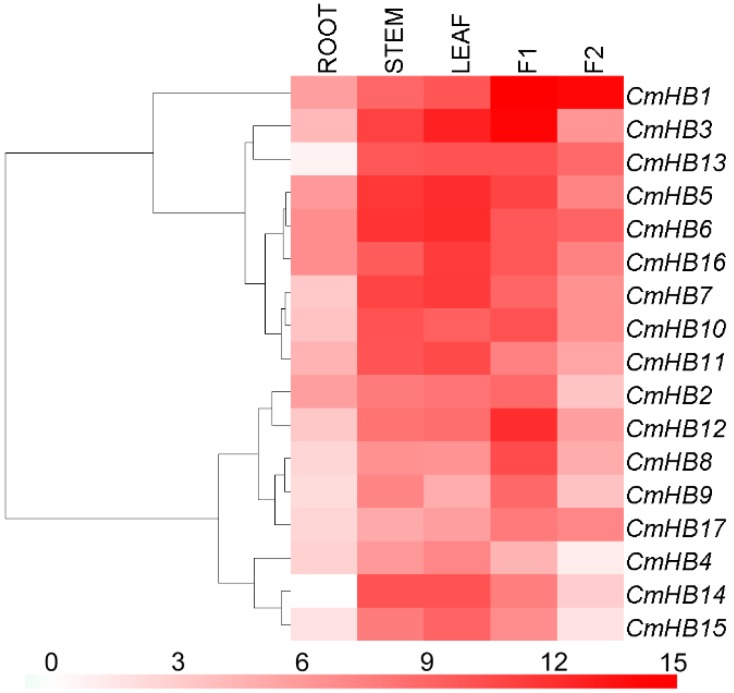
Because HD-Zip factors in chrysanthemum have not been documented previously, we investigated the expression profiles of these genes. The results showed differential expression of the 17 CmHB genes throughout the plant (Figure 3). However, the expression of CmHB1 in the tubular florets was more than three orders of magnitude higher than that of CmHB14 in the roots. Interestingly, the CmHB5 and CmHB16 paralogs exhibited a similar expression pattern, whereas the CmHB13 and CmHB14 paralogs showed different patterns.
Figure 3.
Differential transcription of CmHB genes. F1, tubular florets; F2, ray florets at the budding stage. White and red indicate lower and higher transcript abundance, respectively. The relative expression levels of each CmHB gene were log2 transformed, the lowest expression level was defined as control (zero).
3.5. Expression of CmHB Genes After Treatment with Phytohormones
CmHB7 was significantly induced after 4 h of ABA treatment, although CmHB3, 11, and 12 were repressed at 1 h. The CmHB2 genes were down-regulated by exogenous ABA at 4 h, although CmHB6 and CmHB17 were induced at 24 h. In addition, the CmHB1, 5, 8, 9, 10, and 16 transcripts decreased at 1 h and/or 4 h but increased at 12 h and/or 24 h after ABA treatment, whereas CmHB4, 13, 14, and 15 expression was not affected by ABA (Figure 4a). CmHB3, 4, 7, 10 and 12 were strongly down-regulated by MeJA treatment, whereas CmHB8, 13 and 14 were only slightly down-regulated. Although the expression of the other CmHBs (1, 2, 6, 11, 15, 16 and 17) was not significantly altered by MeJA treatment, CmHB5 and CmHB9 were induced at 4 h and 14 h, respectively (Figure 4b). After SA treatment, CmHB4 and CmHB12 were repressed at 1 h and 12 h, whereas CmHB11 was repressed only at 12 h; however, CmHB7, 9 and 12 were not significantly altered. Moreover, the expression levels of the other eleven CmHBs increased at 4 h after SA treatment, whereas the levels of CmHB2, 3, 8, 13 and 14 decreased at 12 h (Figure 4c).
Figure 4.
Differential transcription of CmHB genes in leaves induced by the exogenous supply of (a) abscisic acid (ABA), (b) methyl jasmonate (MeJA), (c) salicylic acid (SA), (d) salinity stress, (e) osmotic stress, and (f) wound treatments. Blue and yellow indicate lower and higher transcript abundance compared with the relevant controls, respectively. Grey blocks indicate that the transcription was not detected.
3.6. Expression Profiling of CmHB Genes Under Abiotic Stress
Three main expression patterns of the CmHB genes were observed under salinity stress. Although the expression of CmHB3, 5, 6, and 11 was not significantly altered by the NaCl treatment, CmHB1, 8, 9 and 17 were strongly induced at 24 h, whereas the other nine CmHB genes were slightly up-regulated (Figure 4d). Eight CmHB genes (2, 3, 4, 5, 6, 10, 13, and 16) were weakly regulated by drought stress and presented a less than 2-fold range of variation. CmHB9 and CmHB17 were markedly induced at 1 h after PEG treatment, whereas four CmHB genes (1, 7, 8 and 12) were induced at 12 h. Furthermore, CmHB11, 14 and 15 were down-regulated by high osmotic pressure at 12 h (Figure 4e). Three CmHB genes (4, 10, and 11) were significantly repressed by mechanical damage, whereas the transcription of four CmHB genes (1, 7, 8, and 12) was significantly increased. In addition, CmHB3, 9, 16 and 17 were slightly up-regulated by mechanical damage, whereas the expression of the other 6 CmHBs was not significantly altered (Figure 4f).
4. Discussion
The HD-Zip gene family has been isolated and characterized in certain plant species, including Arabidopsis [3], poplar [19], soybean [15], and rice [10]. However, this family has not previously been studied in chrysanthemum. In the current study, we performed an overall analysis of the HD-Zip gene family in the chrysanthemum transcriptome, including an analysis of their phylogeny, conserved motifs and expression profiles. The comparative analysis of the HD-Zip family in Arabidopsis and chrysanthemum allowed for the prediction of various functions of the chrysanthemum HD-Zip family members and helped to facilitate further gene function analysis.
4.1. Comparative Analysis of the Chrysanthemum and Arabidopsis HD-Zip Gene Families
In this study, a total of 17 full-length HD-Zip genes were identified in chrysanthemum based on transcriptome data. A comparative analysis of the chrysanthemum and Arabidopsis HD-Zip genes found that seventeen CmHB genes distributed in the HD-Zip subfamilies I and II, whereas none were distributed in subfamilies III and IV (Figure 1). This observation may have been caused by limitations of the transcriptome data, which means the expression of III and IV subfamily gene was too low to be detected. All the CmHBs were classified by the presence of a highly conserved homeodomain (Figure 2). In addition, the assessment of the subcellular localization of chrysanthemum HD-Zip proteins revealed strong support for their functional roles in in the regulation of transcription (Table 1). Nonetheless, the transcriptional activity of these family members required additional investigation.
We further analyzed the conserved motifs among the chrysanthemum HD-Zip family members using the MEME program and found that the majority of CmHBs within the same group shared similar motifs (Figure 2), suggesting that these conserved motifs play crucial roles in group-specific functions. However, a high divergence in structure was found among the different groups. As the motif analysis in pear HD-Zip gene family, subfamily III contains the most motifs, whereas subfamilies I and II contain the fewest motifs [40]. Only 7 putative motifs with E values less than 1e-3 were identified in subfamilies I and II, and the position of the motifs among the members of an individual subfamily was conserved. These two features of chrysanthemum are consistent with those of other species [40].
Previous studies suggested that tandem and segmental duplications play a substantial role in the expansion of gene families during the process of genome evolution [11]. Each chrysanthemum HD-Zip subfamily presented a unique motif and a similar motif composition, which suggests that the gene family might have expanded by duplication.
4.2. miRNA Target Site Prediction
In Arabidopsis, HD-Zip III subfamily genes are the targets of two miRNAs, miRNA165 and miRNA166, and this characteristic is conserved in other plants as well [1]. However, reports of miRNA with HD-Zip I and II subfamily interactions are rare. According to our predictions, 8 CmHB genes should be targeted by 10 miRNA families (Table 2), which suggests that other interaction modules might occur in the HD-Zip I and II subfamilies. However, the regulation pathways for these interactions in plants should be verified by further research.
4.3. Expression Patterns of CmHB Genes
Because gene expression patterns can provide important clues for gene functions, we employed qRT-PCR to examine the expression of the CmHB genes in the roots, stems and leaves of young seedlings as well as in the tube and ray florets of inflorescences at the bud stage (Figure 3). The expression profiles revealed spatial variations of CmHB expression in different organs. Furthermore, a pair of paralogous genes (CmHB5 and CmHB16) exhibited distinct expression patterns, suggesting that significant functional divergence might have occurred following the duplication events [59].
AtHB1 is highly expressed throughout the pavement, basal and trichome cells of the mature leaves during leaf development [60], and its orthologue in chrysanthemum, CmHB6, was also expressed at the highest level in leaves (Figure 3). Furthermore, the other homologs of AtHB1, CmHB3, 5, and 16 were highly expressed in the leaves (Figure 3), suggesting they may have functional redundancy in leaf morphogenesis. AtHB4 and HAT3, two class II HD-ZIP transcription factors, also control leaf development in Arabidopsis, and their closest homolog CmHB4 has the highest expression level in the leaves among different organs (Figure 3), which implies that these genes have not yet undergone functional divergence. However, additional research is required to determine the functions of these CmHB genes.
The alignment of full-length protein sequences have confirmed that the HD-Zip II proteins can be distributed into two subfamilies, one consisting of HAT22 and HAT9 and the other consisting of HATI, AtHB4, HAT3 and AtHB2 [61]. Expression studies using microarrays have shown that HAT22 expression is up-regulated during drought in Arabidopsis [62]. However, there is little functional evidence to suggest a role for HD-Zip II TFs in response to water deficit [63]. The homolog of Arabidopsis HAT22 in Medicago truncatula, MtHB2, was induced by drought and salt stresses, and it functioned as a negative regulator in plant drought and salt tolerance [16]. The homologs of MtHB2 in chrysanthemum, CmHB8 and CmHB9, had similar expression patterns and were also induced by drought and salt stress (Figure 4).
ABA is extensively involved in the response to various biotic and abiotic stresses, including pathogen infection, cold, and osmotic stress [64]. AtHB7 and AtHB12 are strongly induced by water-deficit and ABA, and they exhibit essential functions as mediators of a negative feedback effect on ABA signaling in the plant response to water deficit [65]. The closest homologs of AtHB7 in chrysanthemum, CmHB1 and CmHB7, were also significantly induced by ABA and osmotic stress treatments (Figure 4a,e), suggesting that they may have similar functions in the chrysanthemum. However, the homolog of AtHB12, CmHB12, was induced by osmotic stress but suppressed by ABA, suggesting that there may be functional divergence between AtHB12 and CmHB12. AtHB6 is expressed constitutively in the seedlings but significantly up-regulated in the seedlings subjected to water deficit, osmotic stress or exogenous treatment with ABA [66]. However, the expression of CmHB15 was not affected by ABA, and it was down-regulated by high osmotic pressure at 12 h (Figure 4). This response suggests that CmHB15 may have a distant phylogenetic relationship with AtHB6, and this relationship was also implied by phylogenetic analysis (Figure 1).
SA and MeJA present coordinated functions in biotic stress signaling upon pathogen infection by activating the transcription of several defense-related genes [67]; however, no report has focused on the responses of the Arabidopsis HD-Zip gene family members to the two hormones. Helianthus annuus (sunflower) HAHB4 is induced by JA, wounding and insect attack, and can negatively regulating SA accumulation [41]. Its homologs in chrysanthemum, CmHB1 and CmHB7, were strongly induced by wounding, but not by JA. Furthermore, its homologs in Arabidopsis, AtHB7 and AtHB12, mediate a growth response to water deficit [68]. We therefore suggested CmHB1 and CmHB7 may experience neofunctionalization, with respect to HAHB4. We investigated the responses of CmHB genes to SA and MeJA, and the results showed that the CmHB genes were up- or down-regulated by the exogenous supply of hormones (Figure 4b,c), thus indicating that CmHB genes may be involved in responses to various plant hormones that elicit a stress response.
5. Conclusions
To our knowledge, this study is the first transcriptome-wide analysis of the HD-Zip family in chrysanthemum. The changes in expression of 17 CmHB genes in response to a range of phytohormones and abiotic stress treatments were characterized. These findings lay the foundation for future research into the function of CmHB genes in plant stress responses, which will promote their application in chrysanthemum breeding.
Acknowledgments
This study was funded by the National Science Fund for Distinguished Young Scholars (31425022), the New agricultural varieties, technology and new model update project of Jiangsu Province (SXGC(2015)322), National Natural Science Foundation of China (31501792, 31301809), Special Fund for Agro-scientific Research in the Public Interest (201403039), the Natural Science Fund of Jiangsu Province (BK20150657), the China Postdoctoral Science Foundation (2014M561673, 2015T80564), and the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education.
Supplementary Materials
The following are available online at www.mdpi.com/2073-4425/7/5/19/s1, Table S1: Primer sequences used to perform 3’- RACE PCR, Table S2: Primer sequences used to amplify the open reading frames of 17 CmHB genes, Table S3: Primer sequences used for the transcription analysis of the 17 CmHB genes.
Author Contributions
Aiping Song, Zhiyong Guan and Tianwei Gao conceived and designed the experiments; Qingqing Fan, Jingjing Xin and Kunkun Zhao performed the experiments; Aiping Song and Dan Wu analyzed the data; Fadi Chen, Sumei Chen and Zhiyong Guan contributed reagents/materials/analysis tools; Aiping Song and Peiling Li wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ariel F.D., Manavella P.A., Dezar C.A., Chan R.L. The true story of the HD-Zip family. Trends Plant Sci. 2007;12:419–426. doi: 10.1016/j.tplants.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Zalewski C.S., Floyd S.K., Furumizu C., Sakakibara K., Stevenson D.W., Bowman J.L. Evolution of the class IV HD-zip gene family in streptophytes. Mol. Biol. Evol. 2013;30:2347–2365. doi: 10.1093/molbev/mst132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schena M., Davis R.W. HD-Zip proteins: members of an Arabidopsis homeodomain protein superfamily. Proc. Natl. Acad. Sci. USA. 1992;89:3894–3898. doi: 10.1073/pnas.89.9.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elhiti M., Stasolla C. Structure and function of homodomain-leucine zipper (HD-Zip) proteins. Plant Signal. Behav. 2009;4:86–88. doi: 10.4161/psb.4.2.7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palena C.M., Tron A.E., Bertoncini C.W., Gonzalez D.H., Chan R.L. Positively charged residues at the N-terminal arm of the homeodomain are required for efficient DNA binding by homeodomain-leucine zipper proteins. J. Mol. Biol. 2001;308:39–47. doi: 10.1006/jmbi.2001.4563. [DOI] [PubMed] [Google Scholar]
- 6.Tron A.E., Comelli R.N., Gonzalez D.H. Structure of homeodomain-leucine zipper/DNA complexes studied using hydroxyl radical cleavage of DNA and methylation interference. Biochemistry. 2005;44:16796–16803. doi: 10.1021/bi0513150. [DOI] [PubMed] [Google Scholar]
- 7.Sessa G., Steindler C., Morelli G., Ruberti I. The Arabidopsis Athb-8, -9 and genes are members of a small gene family coding for highly related HD-ZIP proteins. Plant Mol. Biol. 1998;38:609–622. doi: 10.1023/A:1006016319613. [DOI] [PubMed] [Google Scholar]
- 8.Abe M., Katsumata H., Komeda Y., Takahashi T. Regulation of shoot epidermal cell differentiation by a pair of homeodomain proteins in Arabidopsis. Development. 2003;130:635–643. doi: 10.1242/dev.00292. [DOI] [PubMed] [Google Scholar]
- 9.Ciarbelli A.R., Ciolfi A., Salvucci S., Ruzza V., Possenti M., Carabelli M., Fruscalzo A., Sessa G., Morelli G., Ruberti I. The Arabidopsis homeodomain-leucine zipper II gene family: diversity and redundancy. Plant Mol. Biol. 2008;68:465–478. doi: 10.1007/s11103-008-9383-8. [DOI] [PubMed] [Google Scholar]
- 10.Agalou A., Purwantomo S., Övernäs E., Johannesson H., Zhu X., Estiati A., de Kam R.J., Engström P., Slamet-Loedin I.H., Zhu Z., et al. A genome-wide survey of HD-Zip genes in rice and analysis of drought-responsive family members. Plant Mol. Biol. 2008;66:87–103. doi: 10.1007/s11103-007-9255-7. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y., Zhou Y., Jiang H., Li X., Gan D., Peng X., Zhu S., Cheng B. Systematic analysis of sequences and expression patterns of drought-responsive members of the HD-Zip gene family in maize. PLoS ONE. 2011;6:19. doi: 10.1371/journal.pone.0028488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Re D.A., Dezar C.A., Chan R.L., Baldwin I.T., Bonaventure G. Nicotiana attenuata NaHD20 plays a role in leaf ABA accumulation during water stress, benzylacetone emission from flowers, and the timing of bolting and flower transitions. J. Exp. Bot. 2011;62:155–166. doi: 10.1093/jxb/erq252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z., Chen X., Guan X., Liu Y., Chen H., Wang T., Mouekouba L.D., Li J., Wang A. A genome-wide survey of homeodomain-leucine zipper genes and analysis of cold-responsive HD-Zip I members’ expression in tomato. Biosci. Biotechnol. Biochem. 2014;78:1337–1349. doi: 10.1080/09168451.2014.923292. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto T., Morishige H., Tanaka T., Kanamori H., Komatsuda T., Sato K., Itoh T., Wu J., Nakamura S. Transcriptome analysis of barley identifies heat shock and HD-Zip I transcription factors up-regulated in response to multiple abiotic stresses. Mol. Breeding. 2014;34:761–768. doi: 10.1007/s11032-014-0048-9. [DOI] [Google Scholar]
- 15.Chen X., Chen Z., Zhao H., Zhao Y., Cheng B., Xiang Y. Genome-wide analysis of soybean HD-Zip gene family and expression profiling under salinity and drought treatments. PLoS ONE. 2014;9:19. doi: 10.1371/journal.pone.0087156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song S., Chen Y., Zhao M., Zhang W.H. A novel Medicago truncatula HD-Zip gene, MtHB2, is involved in abiotic stress responses. Environ. Exp. Bot. 2012;80:1–9. doi: 10.1016/j.envexpbot.2012.02.001. [DOI] [Google Scholar]
- 17.Rueda E., Dezar C., Gonzalez D., Chan R. Hahb-10, a sunflower homeobox-leucine zipper gene, is involved in the response to dark/light conditions and promotes a reduction of the life cycle when expressed in Arabidopsis. Plant Cell Physiol. 2005;46:1954–1963. doi: 10.1093/pcp/pci210. [DOI] [PubMed] [Google Scholar]
- 18.Sakakibara K., Nishiyama T., Kato M., Hasebe M. Isolation of homeodomain–leucine zipper genes from the moss Physcomitrella patens and the evolution of homeodomain–leucine zipper genes in land plants. Mol. Biol. Evol. 2001;18:491–502. doi: 10.1093/oxfordjournals.molbev.a003828. [DOI] [PubMed] [Google Scholar]
- 19.Hu R., Chi X., Chai G., Kong Y., He G., Wang X., Shi D., Zhang D., Zhou G. Genome-wide identification, evolutionary expansion, and expression profile of homeodomain-leucine zipper gene family in poplar (Populus trichocarpa) PloS ONE. 2012;7:19. doi: 10.1371/journal.pone.0031149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henriksson E., Olsson A.S., Johannesson H., Johansson H., Hanson J., Engström P., Söderman E. Homeodomain leucine zipper class I genes in Arabidopsis. Expression patterns and phylogenetic relationships. Plant Physiol. 2005;139:509–518. doi: 10.1104/pp.105.063461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoyama T., Dong C.H., Wu Y., Carabelli M., Sessa G., Ruberti I., Morelli G., Chua N.H. Ectopic expression of the Arabidopsis transcriptional activator Athb-1 alters leaf cell fate in tobacco. Plant Cell. 1995;7:1773–1785. doi: 10.1105/tpc.7.11.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Y., Ma Q., Jin X., Peng X., Liu J., Deng L., Yan H., Sheng L., Jiang H., Cheng B. A novel maize homeodomain–leucine zipper (HD-Zip) I gene, Zmhdz10, positively regulates drought and salt tolerance in both rice and arabidopsis. Plant Cell Physiol. 2014;55:1142–1156. doi: 10.1093/pcp/pcu054. [DOI] [PubMed] [Google Scholar]
- 23.De Smet I., Lau S., Ehrismann J.S., Axiotis I., Kolb M., Kientz M., Weijers D., Jürgens G. Transcriptional repression of BODENLOS by HD-ZIP transcription factor HB5 in Arabidopsis thaliana. J. Exp. Bot. 2013;64:3009–3019. doi: 10.1093/jxb/ert137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakuma S., Pourkheirandish M., Hensel G., Kumlehn J., Stein N., Tagiri A., Yamaji N., Ma J.F., Sassa H., Koba T. Divergence of expression pattern contributed to neofunctionalization of duplicated HD-Zip I transcription factor in barley. New Phytol. 2013;197:939–948. doi: 10.1111/nph.12068. [DOI] [PubMed] [Google Scholar]
- 25.Turchi L., Baima S., Morelli G., Ruberti I. Interplay of HD-Zip II and III transcription factors in auxin-regulated plant development. J. Exp. Bot. 2015;66:5043–5053. doi: 10.1093/jxb/erv174. [DOI] [PubMed] [Google Scholar]
- 26.Sawa S., Ohgishi M., Goda H., Higuchi K., Shimada Y., Yoshida S., Koshiba T. The HAT2 gene, a member of the HD-Zip gene family, isolated as an auxin inducible gene by DNA microarray screening, affects auxin response in Arabidopsis. Plant J. 2002;32:1011–1022. doi: 10.1046/j.1365-313X.2002.01488.x. [DOI] [PubMed] [Google Scholar]
- 27.Reymond M.C., Brunoud G., Chauvet A., Martínez-Garcia J.F., Martin-Magniette M.L., Monéger F., Scutt C.P. A light-regulated genetic module was recruited to carpel development in Arabidopsis following a structural change to SPATULA. Plant Cell. 2012;24:2812–2825. doi: 10.1105/tpc.112.097915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bou-Torrent J., Salla-Martret M., Brandt R., Musielak T., Palauqui J.C., Martínez-García J.F., Wenkel S. ATHB4 and HAT3, two class II HD-ZIP transcription factors, control leaf development in Arabidopsis. Plant Signal. Behav. 2012;7:1382–1387. doi: 10.4161/psb.21824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turchi L., Carabelli M., Ruzza V., Possenti M., Sassi M., Peñalosa A., Sessa G., Salvi S., Forte V., Morelli G. Arabidopsis HD-Zip II transcription factors control apical embryo development and meristem function. Development. 2013;140:2118–2129. doi: 10.1242/dev.092833. [DOI] [PubMed] [Google Scholar]
- 30.Valdes A., Roberts C., Carlsbecker A. HD-Zip class III transcription factors control root development through the modulation of ROS levels. Biotechnologia. 2013;94:217–229. [Google Scholar]
- 31.Côté C.L., Boileau F., Roy V., Ouellet M., Levasseur C., Morency M.J., Cooke J.E., Séguin A., MacKay J.J. Gene family structure, expression and functional analysis of HD-Zip III genes in angiosperm and gymnosperm forest trees. BMC Plant Biol. 2010 doi: 10.1186/1471-2229-10-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baima S., Possenti M., Matteucci A., Wisman E., Altamura M.M., Ruberti I., Morelli G. The Arabidopsis ATHB-8 HD-zip protein acts as a differentiation-promoting transcription factor of the vascular meristems. Plant Physiol. 2001;126:643–655. doi: 10.1104/pp.126.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robischon M., Du J., Miura E., Groover A. The Populus class III HD ZIP, popREVOLUTA, influences cambium initiation and patterning of woody stems. Plant Physiol. 2011;155:1214–1225. doi: 10.1104/pp.110.167007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vernoud V., Laigle G., Rozier F., Meeley R.B., Perez P., Rogowsky P.M. The HD-ZIP IV transcription factor OCL4 is necessary for trichome patterning and anther development in maize. Plant J. 2009;59:883–894. doi: 10.1111/j.1365-313X.2009.03916.x. [DOI] [PubMed] [Google Scholar]
- 35.Yu L., Chen X., Wang Z., Wang S., Wang Y., Zhu Q., Li S., Xiang C. Arabidopsis enhanced drought tolerance1/HOMEODOMAIN GLABROUS11 confers drought tolerance in transgenic rice without yield penalty. Plant Physiol. 2013;162:1378–1391. doi: 10.1104/pp.113.217596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamata N., Okada H., Komeda Y., Takahashi T. Mutations in epidermis-specific HD-ZIP IV genes affect floral organ identity in Arabidopsis thaliana. Plant J. 2013;75:430–440. doi: 10.1111/tpj.12211. [DOI] [PubMed] [Google Scholar]
- 37.Ogawa E., Yamada Y., Sezaki N., Kosaka S., Kondo H., Kamata N., Abe M., Komeda Y., Takahashi T. ATML1 and PDF2 play a redundant and essential role in Arabidopsis embryo development. Plant Cell Physiol. 2015;56:1183–1192. doi: 10.1093/pcp/pcv045. [DOI] [PubMed] [Google Scholar]
- 38.Khosla A., Boehler A.P., Bradley A.M., Neumann T.R., Schrick K. HD-Zip proteins GL2 and HDG11 have redundant functions in Arabidopsis trichomes, and GL2 activates a positive feedback loop via MYB23. Plant Cell. 2014;26:2184–2200. doi: 10.1105/tpc.113.120360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dezar C.A., Giacomelli J.I., Manavella P.A., Ré D.A., Alves-Ferreira M., Baldwin I.T., Bonaventure G., Chan R.L. HAHB10, a sunflower HD-Zip II transcription factor, participates in the induction of flowering and in the control of phytohormone-mediated responses to biotic stress. J. Exp. Bot. 2011;62:1061–1076. doi: 10.1093/jxb/erq339. [DOI] [PubMed] [Google Scholar]
- 40.Wang H., Lin J., Li X.G., Chang Y. Genome-wide identification of pear HD-Zip gene family and expression patterns under stress induced by drought, salinity, and pathogen. Acta Physiol. Plant. 2015;37:1–19. doi: 10.1007/s11738-014-1746-y. [DOI] [Google Scholar]
- 41.Manavella P.A., Dezar C.A., Bonaventure G., Baldwin I.T., Chan R.L. HAHB4, a sunflower HD-Zip protein, integrates signals from the jasmonic acid and ethylene pathways during wounding and biotic stress responses. Plant J. 2008;56:376–388. doi: 10.1111/j.1365-313X.2008.03604.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang C., Ma R., Shen Z., Sun X., Korir N., Yu M. Genome-wide analysis of the homeodomain-leucine zipper (HD-ZIP) gene family in peach (Prunus persica) Genet. Mol. Res. 2014;13:2654–2668. doi: 10.4238/2014.April.8.8. [DOI] [PubMed] [Google Scholar]
- 43.Belamkar V., Weeks N.T., Bharti A.K., Farmer A.D., Graham M.A., Cannon S.B. Comprehensive characterization and RNA-Seq profiling of the HD-Zip transcription factor family in soybean (Glycine max) during dehydration and salt stress. BMC Genom. 2014 doi: 10.1186/1471-2164-15-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.An J., Song A., Guan Z., Jiang J., Chen F., Lou W., Fang W., Liu Z., Chen S. The over-expression of Chrysanthemum crassum CcSOS1 improves the salinity tolerance of chrysanthemum. Mol. Biol. Rep. 2014;41:4155–4162. doi: 10.1007/s11033-014-3287-2. [DOI] [PubMed] [Google Scholar]
- 45.Song A., Lu J., Jiang J., Chen S., Guan Z., Fang W., Chen F. Isolation and characterisation of Chrysanthemum crassum SOS1, encoding a putative plasma membrane Na+/H+ antiporter. Plant Biol. 2012;14:706–713. doi: 10.1111/j.1438-8677.2011.00560.x. [DOI] [PubMed] [Google Scholar]
- 46.Song A., An J., Guan Z., Jiang J., Chen F., Lou W., Fang W., Liu Z., Chen S. The constitutive expression of a two transgene construct enhances the abiotic stress tolerance of chrysanthemum. Plant Physiol. Biochem. 2014;80:114–120. doi: 10.1016/j.plaphy.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 47.Song A., Li P., Jiang J., Chen S., Li H., Zeng J., Shao Y., Zhu L., Zhang Z., Chen F. Phylogenetic and transcription analysis of chrysanthemum WRKY transcription factors. Int. J. Mol. Sci. 2014;15:14442–14455. doi: 10.3390/ijms150814442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang F., Wang Z., Dong W., Sun C., Wang H., Song A., He L., Fang W., Chen F., Teng N. Transcriptomic and proteomic analysis reveals mechanisms of embryo abortion during chrysanthemum cross breeding. Sci. Rep. 2014 doi: 10.1038/srep06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 51.Horton P., Park K.J., Obayashi T., Fujita N., Harada H., Adams-Collier C., Nakai K. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 2007;35:585–587. doi: 10.1093/nar/gkm259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bailey T.L., Johnson J., Grant C.E., Noble W.S. The MEME Suite. Nucleic Acids Res. 2015 doi: 10.1093/nar/gkv416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitchell A., Chang H.Y., Daugherty L., Fraser M., Hunter S., Lopez R., McAnulla C., McMenamin C., Nuka G., Pesseat S. The InterPro protein families database: the classification resource after 15 years. Nucleic Acids Res. 2014 doi: 10.1093/nar/gku1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dai X., Zhao P.X. psRNATarget: A plant small RNA target analysis server. Nucleic Acids Res. 2011;39:155–159. doi: 10.1093/nar/gkr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rozen S., Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 56.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 57.de Hoon M.J., Imoto S., Nolan J., Miyano S. Open source clustering software. Bioinformatics. 2004;20:1453–1454. doi: 10.1093/bioinformatics/bth078. [DOI] [PubMed] [Google Scholar]
- 58.Eisen M.B., Spellman P.T., Brown P.O., Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song A., Wu D., Fan Q., Tian C., Chen S., Guan Z., Xin J., Zhao K., Chen F. Transcriptome-wide identification and expression profiling analysis of chrysanthemum trihelix transcription factors. Int. J. Mol. Sci. 2016 doi: 10.3390/ijms17020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schliep M., Ebert B., Simon-Rosin U., Zoeller D., Fisahn J. Quantitative expression analysis of selected transcription factors in pavement, basal and trichome cells of mature leaves from Arabidopsis thaliana. Protoplasma. 2010;241:29–36. doi: 10.1007/s00709-009-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morelli G., Baima S., Carabelli M., di Cristina M., Lucchetti S., Sessa G., Steindler C., Ruberti I. Cellular Integration of Signalling Pathways in Plant Development. Springer; Berlin, Germany: 1998. Homeodomain-leucine zipper proteins in the control of plant growth and development; pp. 251–262. [Google Scholar]
- 62.Huang D., Wu W., Abrams S.R., Cutler A.J. The relationship of drought-related gene expression in Arabidopsis thaliana to hormonal and environmental factors. J. Exp. Bot. 2008;59:2991–3007. doi: 10.1093/jxb/ern155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harris J.C., Hrmova M., Lopato S., Langridge P. Modulation of plant growth by HD-Zip class I and II transcription factors in response to environmental stimuli. New Phytol. 2011;190:823–837. doi: 10.1111/j.1469-8137.2011.03733.x. [DOI] [PubMed] [Google Scholar]
- 64.Mittler R., Blumwald E. The roles of ROS and ABA in systemic acquired acclimation. Plant Cell. 2015;27:64–70. doi: 10.1105/tpc.114.133090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valdés A.E., Övernäs E., Johansson H., Rada-Iglesias A., Engström P. The homeodomain-leucine zipper (HD-Zip) class I transcription factors ATHB7 and ATHB12 modulate abscisic acid signalling by regulating protein phosphatase 2C and abscisic acid receptor gene activities. Plant Mol. Biol. 2012;80:405–418. doi: 10.1007/s11103-012-9956-4. [DOI] [PubMed] [Google Scholar]
- 66.Söderman E., Hjellström M., Fahleson J., Engström P. The HD-Zip gene ATHB6 in Arabidopsis is expressed in developing leaves, roots and carpels and up-regulated by water deficit conditions. Plant Mol. Biol. 1999;40:1073–1083. doi: 10.1023/A:1006267013170. [DOI] [PubMed] [Google Scholar]
- 67.Vos I.A., Moritz L., Pieterse C.M., Van Wees S.C. Impact of hormonal crosstalk on plant resistance and fitness under multi-attacker conditions. Front Plant Sci. 2015 doi: 10.3389/fpls.2015.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olsson A.S., Engström P., Söderman E. The homeobox genes ATHB12 and ATHB7 encode potential regulators of growth in response to water deficit in Arabidopsis. Plant Mol. Biol. 2004;55:663–677. doi: 10.1007/s11103-004-1581-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.