Abstract
This article presents initial diagnostic workup and criteria for diagnosing solitary plasmacytoma of bone (SPB) versus multiple myeloma. The authors discuss the incorporation of current imaging technologies into the diagnosis and staging of SPB and multiple myeloma. In addition, the article addresses treatment modalities and discusses the importance of oncology nurses’ awareness of this rare condition.
Case Study
Mr. J is a 44-year-old African American patient with a chief complaint of “low back pain.” He presented as a follow-up to his initial appointment four weeks prior for back pain. He stated that his back pain had decreased from a level of 5 to 3 on most days, but he still had pain, particularly in the evening.
Mr. J had had three sinus infections with antibiotic treatment in the past year. He has no known chronic medical conditions. At 74 inches tall and weighing 180 lbs., he follows a vegan diet and exercises five to seven days per week. Mr. J’s focused physical examination found him to be alert with no acute distress. His spinal examination showed paraspinal tenderness in the lumbar (or L-S) region and forward flexion and extension without limitation, with no lesions noted. Mr. J’s deep tendon reflexes scored normal at +2, symmetric; his muscle strength also was normal at +5/5.
The healthcare team planned to take an x-ray of Mr. J’s lumbar spine and continue nonsteroidal medication. Follow-up would occur in two to three weeks if no improvement was noted or sooner if symptoms increased or x-ray abnormalities were found. X-ray revealed a single focal osteolytic lesion in the lumbar vertebrae. With a differential diagnosis of solitary plasmacytoma of bone (SPB), the team planned to do a workup to rule out multiple myeloma.
Diagnostic Evaluation
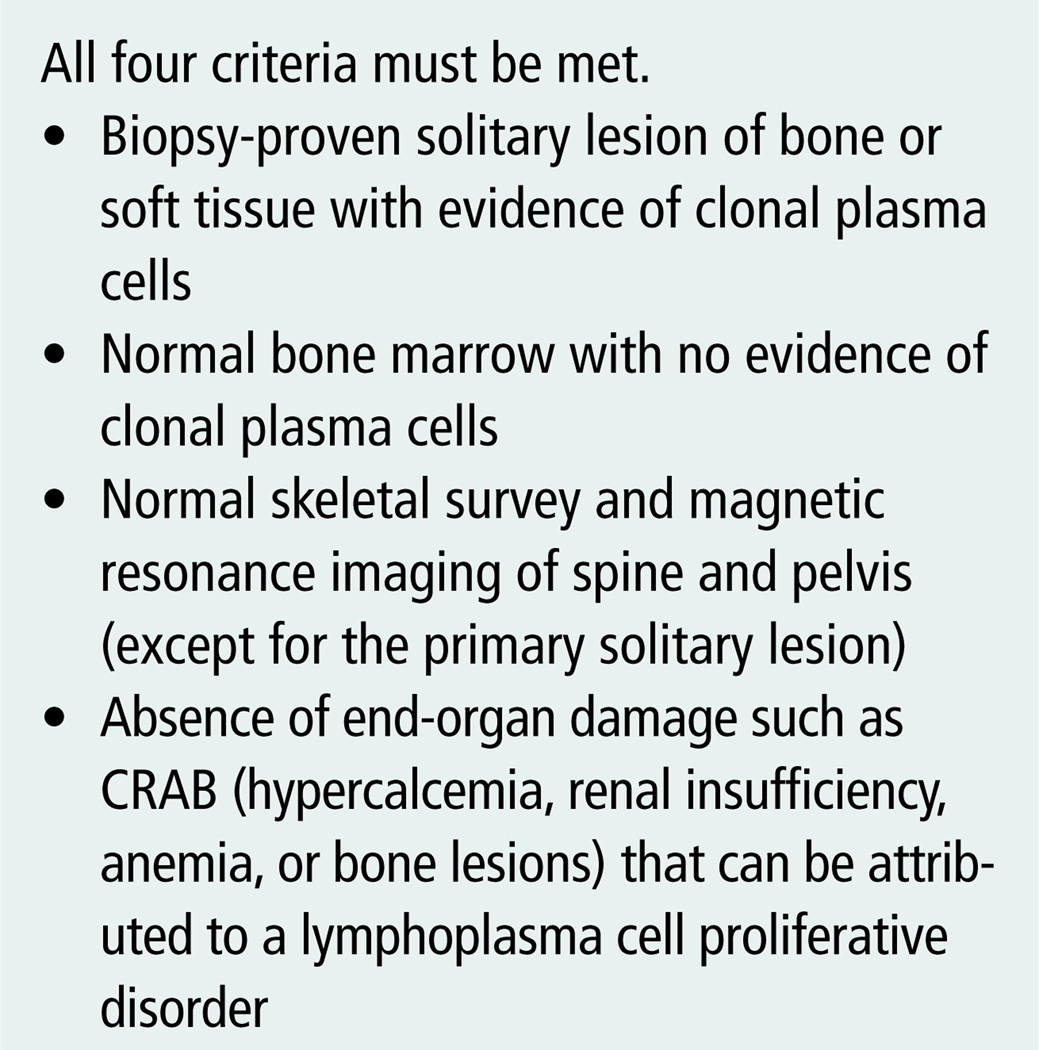
The initial diagnostic workup for SPB requires a number of baseline blood studies, including a complete blood count with differential and platelet count, blood urea nitrogen, serum creatinine and serum electrolytes, serum calcium, albumin, lactate dehydrogenase, beta-2 immunoglobulin, quantitative immunoglobulin levels, serum protein electrophoresis, and serum immunofixation electrophoresis. Baseline urine analyses include 24-hour urine, urine protein electrophoresis, and urine immunofixation electrophoresis (National Comprehensive Cancer Network [NCCN], 2009). Results of the workup can be used to rule out multiple myeloma versus a localized plasmacytoma. Necessary criteria for a diagnosis of plasmacytoma are summarized in Figure 1.
Figure 1. Solitary Plasmacytoma of Bone Diagnostic Criteria.
Note. From “Criteria for Diagnosis, Staging, Risk Stratification and Response Assessment of Multiple Myeloma,” by R.A. Kyle and S.V. Rajkumar, 2009, Leukemia, 23, p. 5. Copyright 2009 by Macmillan Publishers Limited. Reprinted with permission.
Mild hemolytic anemia is seen with the systemic disorder multiple myeloma, although hemoglobin levels remain in the normal range in SPB. Bone damage can result in calcium mobilization from the affected bone into the serum, leading to hypercalcemia. The serum calcium alteration is seen more frequently in multiple myeloma, with serum calcium levels generally remaining within the normal range in SPB. Elevated creatinine and blood urea nitrogen are indicative of decreased kidney function and often are seen in multiple myeloma; the renal involvement is not present in SPB (DeFilippo et al., 2008).
In addition to testing for serum protein level, a 24-hour urine specimen is collected and tested for total protein. High levels of monoclonal protein in serum and urine are indicative of multiple myeloma. SPB typically is characterized by absent or low serum or urinary levels of monoclonal protein. Although elevated levels of monoclonal protein are seen in 24%–72% of patients with SPB, the levels are much lower than those seen in patients with multiple myeloma (DeFilippo et al., 2008).
Clonal plasma cells produce monoclonal immunoglobulin, which may appear as a monoclonal spike on serum electrophoresis. In addition, an assay for serum immunoglobulin free light chains allows quantitation of both kappa and lambda light chains that are not bound to intact immunoglobulin molecules, allowing for determination of clonality based on the kappa to lambda ratio. An abnormal free light chain ratio is prognostic for progression from SPB to multiple myeloma; the indicators are seen more frequently in multiple myeloma (Dingli et al., 2009). Although light chains could be produced on SPB, laboratory signs in serum electrophoresis usually are not related to the monoclonal component, and a diagnosis of SPB often is based on specific clinical evaluation of a bone-related symptom (Di Micco & Di Micco, 2005). Levels of uninvolved (normal) immunoglobulins may be depressed in multiple myeloma; preserved levels of uninvolved immunoglobulins in patients with SPB are evidence that tumor load is low (DeFilippo et al., 2008). A high level of the enzyme lactate dehydrogenase is another indicator of high tumor cell burden and is expected to be higher in multiple myeloma. Level of beta-2 microglobulin is indicative of tumor mass, is a standard measure of tumor burden, and is expected to be higher in multiple myeloma (NCCN, 2009).
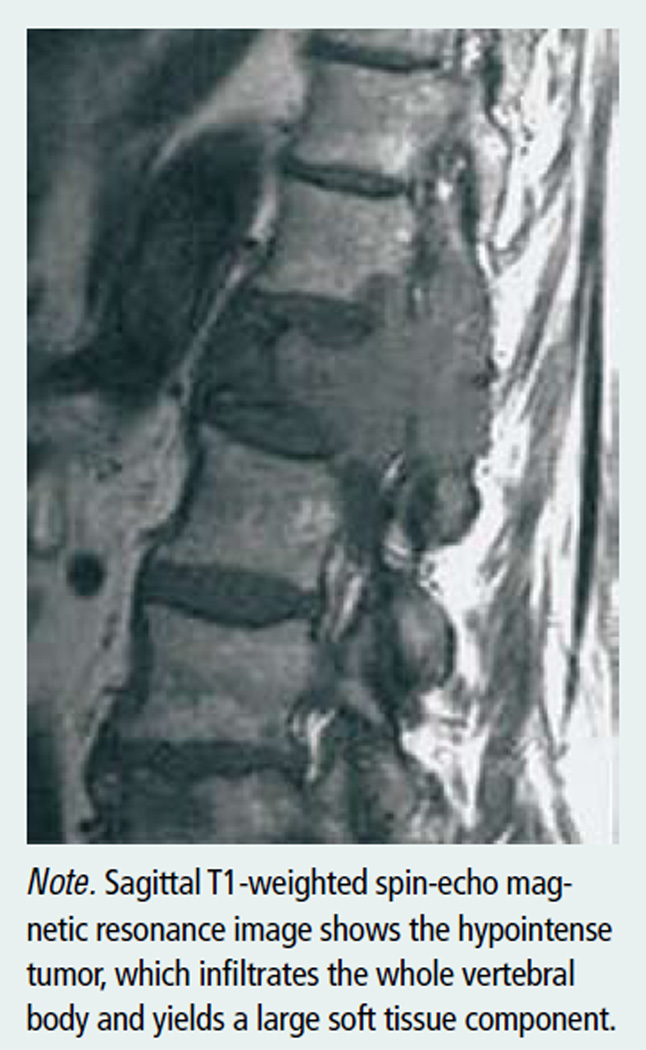
A conventional radiographic survey (x-ray) will show whether the condition is localized to one lesion, indicating SPB (see Figure 2), or whether multiple punched-out lytic lesions are seen diffusely throughout the skeleton, indicating multiple myeloma. After a diagnosis of plasmacytoma has been established, computed tomography scanning or magnetic resonance imaging may be used to define the local extent of the lesion and to aid in radiotherapy treatment planning. Positron-emission scanning has been shown to be useful in the initial staging of SPB and multiple myeloma (Mulligan, 2005). Bone marrow immunohistochemistry can confirm the presence of monoclonal plasma cells and measure plasma cell involvement (NCCN, 2009). Random bone marrow biopsy often is diagnostic of multiple myeloma. In SPB, plasma cell monoclonal proliferation is localized to the affected site; therefore, a random bone marrow biopsy would be negative (Di Micco & Di Micco, 2005).
Figure 2. Solitary Plasmacytoma of Bone With First Lumbar Vertebral Body Involvement.
Note. From Imaging of the Musculoskeletal System (p. 1721) by T. Pope, H.L. Bloem, J. Beltran, W. Morrison, and D.B. Wilson (Eds.), 2008, Philadelphia, PA: Elsevier. Copyright 2008 by Elsevier. Reprinted with permission.
Cytogenetic analysis may be done on serum or bone marrow to look for chromosomal abnormalities. Abnormal karyotypes have been reported in 30%–50% of patients with multiple myeloma and may involve various trisomies and translocations on three or more chromosomes. Chromosomal abnormalities in SPB may be similar to those in multiple myeloma (Mulligan, 2005). Specific chromosomal abnormalities have demonstrated prognostic value; a deletion in chromosome 13 and a translocation between chromosomes 4 and 14 have been associated with a poor prognosis, and translocation between chromosomes 11 and 14 may be associated with improved survival (NCCN, 2009). Other chromosomal abnormalities associated with multiple myeloma and SPB include deletion in chromosome 17 and translocation between chromosomes 14 and 16 (NCCN, 2009).
Staging Systems
To standardize treatment modalities and optimize outcomes for patients with myeloma, the disease must be characterized as clearly as possible at diagnosis. Therefore, staging has been the cornerstone of baseline assessment since the development of the Durie-Salmon system in 1975 (Durie, 2006). The original Durie-Salmon staging system classified patients into one of three stages based on laboratory values, including hemoglobin, serum calcium, monoclonal protein, and creatinine, as well as the number of bone lesions found on x-ray (Greipp et al., 2005). However, with the advent of new imaging technologies, a more comprehensive staging system incorporating this technology should be used. The Durie-Salmon PLUS staging system takes advantage of current imaging systems such as magnetic resonance imaging, whole-body F-18 fluorodeoxy-glucose positron-emission tomography scanning, and whole-body computed tomography scanning to precisely stage the disease with anatomic and functional techniques. The staging system is advantageous in that it provides a means of cell-mass assessment and staging for patients with SPB as well as hyposecretory and nonsecretory myelomas while allowing for improved discernment between the stages of myeloma (Durie, 2006). The system is an invaluable tool in the diagnosis and treatment of patients with plasmacytoma, a disease that cannot be detected with laboratory tests alone.
Another well-validated system for staging is the International Staging System for multiple myeloma. Developers of the International Staging System determined that a combination of serum beta-2 microglobulin and serum albumin provided a simple and powerful means of classification (Greipp et al., 2005). Table 1 summarizes a comparison of criteria for current staging systems.
Table 1.
Comparison of Current Myeloma Staging Systems
| STAGE | DURIE-SALMON PLUSa | INTERNATIONAL STAGING SYSTEMb |
|---|---|---|
| I |
Stage Ia: one focal lesion or plasmacytoma Stage Ib: fewer than five focal lesions; mild diffuse disease |
Serum beta-2 microglobulin lower than 3.5 mg/L Serum albumin 3.5 g/dl or higher |
| II |
Stage IIa and IIb: 5–20 focal lesions; moderate diffuse disease |
Neither stage I nor stage III |
| III |
Stage IIIa and IIIb: more than 20 focal lesions; severe diffuse disease |
Serum beta-2 microglobulin 5.5 mg/L or higher |
From “The Role of Anatomic and Functional Staging in Myeloma: Description of Durie/Salmon PLUS Staging System,” by B.G. Durie, 2006, European Journal of Cancer, 42, p. 1540. Copyright 2006 by Elsevier Limited. Adapted with permission.
From “International Staging System for Multiple Myeloma,” by P.R. Greipp, J.S. Miguel, B.G. Durie, J.J. Crowley, B. Barlogie, J. Blade, … J. Westin, 2005, Journal of Clinical Oncology, 23, p. 3415. Copyright 2005 by the American Society of Clinical Oncology. Adapted with permission.
Treatment
No clear guidelines are available for the treatment of SPB because of the rare nature of the disease. To date, the treatment for patients with SPB is localized radiotherapy. In some cases, surgery for resection may precede radiation (Dagan, Morris, Kirwan, & Mendenhall, 2009). Radiation therapy is reported to be effective in treating patients with SPB, with a local control rate higher than 80% observed when using moderate radiation doses (40–50 Gy) (Dagan et al., 2009).
Despite local control, outcomes vary; about 75% of patients with SPB eventually develop multiple myeloma, with an average time to progression of two to four years (Bhaskar, Gupta, Sharma, Kumar, & Jain, 2009). Persistence of M-protein for longer than one year after radiotherapy appears to be one adverse prognostic element predicting progression to multiple myeloma (Bhaskar et al., 2009).
Controversy exists surrounding the treatment of adjuvant chemotherapy for the prevention of SPB progression to multiple myeloma. Some have reported that adjuvant chemotherapy may delay progression, but others do not consider the treatment to be beneficial (Bhaskar et al., 2009). Chemotherapy’s role in the treatment of patients with SPB is not defined clearly, and most centers reserve its use for patients with progressive disease (Dagan et al., 2009).
In a study by Bhaskar et al. (2009), clonal plasma cells were found to be present at diagnosis in the bone marrow of patients with SPB. The finding may lead to developments in future treatments for patients with SPB.
Discussion
Mr. B received local radiotherapy of the spine and had improved pain control. He was able to continue his job and was not restricted in his activities. He has follow-up appointments every six weeks with his oncologist to perform laboratory tests to rule out progression to multiple myeloma.
Plasmacytomas are clonal proliferations of plasma cells that are cytologically and immunophenotypically identical to plasma cell myeloma but manifest as a localized osseous or extraosseous growth pattern (Dores et al., 2009). This condition is rare; from 1992–2004, 1,543 plasmacytoma and 23,544 multiple myeloma cases were diagnosed among residents of the 12 SEER (Surveillance, Epidemiology, and End Results) areas (Dores et al., 2009). Essentially, two cohorts of patients with plasmacytoma exist—patients who do not progress to systemic disease and patients who develop myeloma (Jawad & Scully, 2009). The cohorts have different overall survival. Patients who do not progress to systemic disease have an overall five-year survival rate of 72%; however, the five-year survival rate for patients who do progress to myeloma is almost the same as in patients who are diagnosed initially with multiple myeloma (25% and 23%, respectively) (Jawad & Scully, 2009).
Although plasmactyomas are rare, the condition has important implications. For primary care providers, awareness and consideration of the condition is important when back pain does not improve with supportive measures or the location of the back pain is in the thoracic spine. Given that back pain is one of the most frequent reasons for patients to seek health care, having familiarity with a range of potential etiologies is important. In addition, oncology nurses, particularly those who work with patients with multiple myeloma, should be familiar with the broad spectrum of symptoms for the holistic care of patients with cancer.
Footnotes
The authors take full responsibility for the content of the article. The authors did not receive honoraria for this work. No financial relationships relevant to the content of this article have been disclosed by the authors or editorial staff.
References
- Bhaskar A, Gupta R, Sharma A, Kumar L, Jain P. Analysis of bone marrow plasma cells in patients with solitary bone plasmacytoma. Cancer Therapy. 2009;7:49–52. Retrieved from http://www.cancer-therapy.org/CT7A/pdf/08._Bhaskar_et_al,_49-52.pdf. [PMC free article] [PubMed] [Google Scholar]
- Dagan R, Morris CG, Kirwan J, Mendenhall WM. Solitary plasmacytoma. American Journal of Clinical Oncology. 2009;32:612–617. doi: 10.1097/COC.0b013e31819cca18. [DOI] [PubMed] [Google Scholar]
- DeFilippo M, Pogliacomi F, Albisinni U, Quintro S, Bocchi C, Sverzellati N, Zompatori M. Occult large epiphyseal solitary plasmacytoma at multidetector row computer tomography detected by magnetic resonance imaging. Acta Biomedica. 2008;79:240–245. [PubMed] [Google Scholar]
- Di Micco P, Di Micco B. Up-date on solitary plasmacytoma and its main differences with multiple myeloma. Experimental Oncology. 2005;27:7–12. [PubMed] [Google Scholar]
- Dingli D, Kyle RA, Rajkumar V, Nowakowski GS, Larson DR, Bida JP, Katzmann JA. Immunoglobulin free light chains and solitary plasmacytoma of bone. Blood. 2009;108:1979–1983. doi: 10.1182/blood-2006-04-015784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dores GM, Landgren O, McGlynn KA, Curtis RE, Linet MS, Devesa SS. Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: Incidence and survival in the United States, 1992–2004. British Journal of Haematology. 2009;144:86–94. doi: 10.1111/j.1365-2141.2008.07421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durie BG. The role of anatomic and functional staging in myeloma: Description of Durie/Salmon PLUS staging system. European Journal of Cancer. 2006;42:1539–1543. doi: 10.1016/j.ejca.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Greipp PR, Miguel JS, Durie BG, Crowley JJ, Barlogie B, Blade J, Westin J. International staging system for multiple myeloma. Journal of Clinical Oncology. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- Jawad M, Scully S. Skeletal plasmacytoma: Progression of disease and impact of local treatment; an analysis of SEER database. Journal of Hematology and Oncology. 2009;2:41. doi: 10.1186/1756-8722-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan ME. Imaging techniques used in the diagnosis, staging, and follow-up of patients with myeloma. Acta Radiologica. 2005;46:716–724. doi: 10.1080/02841850500215360. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology™: Multiple myeloma [v.2.2010] 2009 doi: 10.6004/jnccn.2009.0061. Retrieved from http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PubMed]