ABSTRACT
Islet transplantation has become a widely accepted therapeutic option for selected patients with type 1 diabetes mellitus. However, in order to achieve insulin independence a great number of islets are often pooled from 2 to 4 pancreata donors. Mostly, it is due to the massive loss of islets immediately after transplant. The endothelium plays a key role in the function of native islets and during the revascularization process after islet transplantation. However, if a delayed revascularization occurs, even the remaining islets will also undergo to cell death and late graft dysfunction. Therefore, it is essential to understand how the signals are released from endothelial cells, which might regulate both differentiation of pancreatic progenitors and thereby maintenance of the graft function. New strategies to facilitate islet engraftment and a prompt revascularization could be designed to intervene and might lead to improve future results of islet transplantation.
KEYWORDS: islets function, islet transplantation, revascularization
GROSS ANATOMY OF THE PANCREAS VASCULAR SUPPLY
The pancreas has a rich arterial vascularization received from direct branches of the abdominal aorta (celiac trunk and superior mesenteric artery) with a volume of blood supply about 1% of the cardiac output.1 From above on the superior border, the celiac trunk provides the vascularization then it gives rise to the common hepatic artery and the splenic artery. Thereby, the common hepatic artery supplies the tissues on the right through its branches running toward the head of the pancreas. Meanwhile on the left portion, the splenic artery gives branches to supply arterial blood from the body to tail of the pancreas. From beneath the superior mesenteric artery and its branches supply the pancreas and forms arcades and collateral circulation between both sides.1,2 In general, the main venous circulation of the pancreas drains into the splenic artery and the superior mesenteric vein (SMV). The splenic vein arises in the splenic hilum to the tail of the pancreas and runs from left to right on the posterior surface of the pancreatic body. Then, both the horizontal splenic vein and the vertical SMV form the portal vein.1,2
In situ Vascular Network of the Islets
Microscopically, the pancreatic tissues are composed with approximately 98% acinar and ductal cells and just about 2% of islets cells. The pancreatic islets are clutters, like “islands” of endocrine cells embedded and scattered into enormous amount of the acinar tissues.3 Most of the islets are spheroidal irregular clusters with wide size distribution from 50 - 200 µm, which are composed from 800 – 3,000 cells. However in the context of islet studies and transplantation, it is often considered as 1 islet equivalent (IEQ) the size of 150 µm, and to be formed of 2,500 cells in average.3,4 However, there are significant differences among species, which have to be considered when conducting research using animal models, such as; number of islets, distribution on the pancreata, proportion and integration of each cell lineages into the islets, etc.1
Since the islets contain all lineages of hormone-producing cells, they are the functional units of the endocrine pancreatic tissues. The main populations of islet cells are α-, β-, δ-, pp-, and ϵ-cells, which produce and secrete glucagon, insulin, pancreatic somatostatin, pancreatic polypeptide and ghrelin, respectively, in response to the specific-secretagogues and stimulus.1,2,4 The pancreatic hormones are promptly secreted by the islets into the bloodstream to tightly control the glycaemia in response to the metabolic demands. Therefore, it is due to this key role sustaining a delicate balanced of the metabolic needs that they have a high vascular supply. It is roughly estimated exocrine tissues received 85% while islets received 15% of total pancreas blood flow. Considering these facts, it is then estimated that islets received 10 times more blood supply than exocrine tissues.3
Cellular Structure and Interactions of the Endothelium and Islets
Since the development, blood vessels and islets have close relationship, which initiate the organogenesis of the pancreas through the signals released from the endothelium. In order to achieve it, endothelium regulates the blood flow, angiogenesis, formation and selectivity of the immunological barriers between the blood and the pancreatic tissues.5 The function of the islets is largely influence by the availability of endocrine signals, nutrients and oxygen delivered from theses blood vessels. Due to vigorous metabolic activity, islets mass has a rapid turn over and contain a population of precursors within the islets, which are responsible to maintain the appropriate mass of endocrine cells.1
In addition, each islet receives innervation and a great vascular supply with at least 2 or 3 afferent arterioles that form numerous capillary inside the islet core to delivery all necessary nutrients and oxygen. In the same manner, from the capillaries and small venules the secreted hormones are drained out from the islet and incorporated into the bloodstream.1,2 The endothelium from the islets vessels is formed of fenestrated endothelial cells. These fenestrations are formed in response to the stimulus of vascular endothelial growth factor-A (VEGF-A) and angiopoietin-1 directly secreted by the islet cells.6 On the other hand, endothelial cells secrete fibroblast growth factor-2 (FGF-2) and hepatocyte growth factor (HGF), which has a potent effect on β-cells enhancing their capacity to newly produce and secrete insulin glucose-responsiveness performance (Fig. 1).7-10 Furthermore, when knockout animals for VEGF-A or HGF are generated, they display abnormalities of the islet vessels, have impaired islet function and lose their glucose-stimulated insulin secretion capacities.6-8
FIGURE 1.
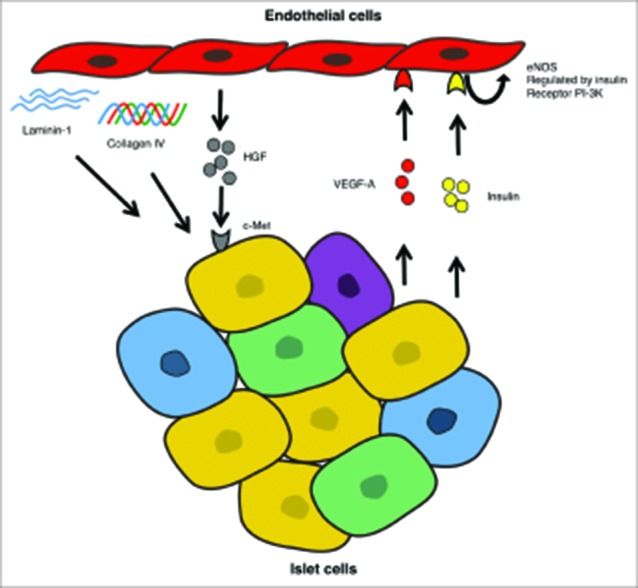
The scheme shows the reciprocal interactions between endothelial cells and islet cells. Insulin mediates the production of nitric oxide (NO) through the regulation of the endothelial nitric oxide synthase (eNOS). VEGF-A induces the formation of both new vessels and their fenestrae on the surface of endothelial cells. HGF released from the endothelial maintain both glucose-stimulated insulin release and β-cell mass by inducing their proliferation. ECMs including laminins, collagens and fibronectin produced by the endothelial cells, promotes β-cell growth, maintenance of their differentiation phenotype and ultimately results in higher insulin production.
The islets and endothelium has also several key interactions through the extracellular matrices (ECM) such collagen I, IV, laminin-1, fibronectin. These ECMs are capable to modulate important epigenetic changes, which include induction of insulin gene expression, stimulate β-cell replication and regulate the secretion of several paracrine factors (Fig. 1). Finally, the endothelium plays also an important role in the regulation of immune responses leading them, to either to autoimmune and alloimmune destruction, otherwise allowing tolerance of β-cells.3
The Role of Vascular Endothelial Cells After Islet Transplantation
Isolated islets are completely devascularized due to the permanent disruption of the vascular supply; under these conditions they are exposed to prolong ischemia. Therefore, if revascularization is delayed, it would result in oxygen and nutrients extend deficit, leading to cell death and graft failure.4,11-13 That is one of the reasons why in the clinical setting, islet transplantations used a large amount islets often pulled from several donors to achieve insulin freedom. It is estimated that only about 40% of the islet will successfully engraft and maintain the graft function over time. The prolong ischemia and delayed revascularization are important factors, which lead most of the islets to graft failure.4,12 Idealistically, the process of revascularization would involve reestablishment of both arterial and venous circulation. However, at least 14 days are required to complete the process of revascularization when no further facilitating interventions are used.12,13
In contrast, when pancreas transplantation is performed these revascularization issues are minor or none. Since both arterial and venous microcirculations have been mostly preserved and major revascularization is surgically completed. Therefore, it is clear that survival, engraftment, and function of the transplanted islets largely depends in how promptly revascularization is completed.4 Naturally, when islets are engrafted into a sinusoidal blood vessel perfusion, and oxygen delivery within the islets would considerably less in comparison to native islets. Revascularization of those islets engraft into the liver may also modify the cellular structure of endothelial cells and ECMs. The newly formed vessels for the islet grafts primarily arise from the endothelial cells residing within the islets and connect to the recipient endothelial becoming chimeric vessels. Interventions with angiogenic factors, such as FGF-2, and VEGF accelerate the process attracting endothelial cells, to induce both proliferation and differentiation to produce a neo-vascular network at the transplantation site.4,6,12 The intra-islet endothelial cells play a key role in the process of revascularization. However, those endothelial cells are particularly fragile and mostly disappear when islet are cultured after isolation. The islet isolation process is extremely hazardous to the endothelial cells and they required special conditions to recover from the stress, which are not compatible to conventional islet culture.
The overcome this major drawback, investigators have sought approach using co-transplant to address this problem. The use of either angiogenic precursors bone marrow-derived or expanded endothelial precursors that will undergo differentiation, and thus contribute to the reestablishment of the vascular network of the transplanted islets.13-15
Recently, an elegant study has shown the use of prevascularized site of transplantation, which is capable to engraft islets. The vascular network established into the subcutaneous tissues was able to engraft islets for >100 days while maintaining their metabolic function comparable to native islets.12
CONCLUSION
The blood vessels provide the islets with signals, nutrients and oxygen, which regulate both islet function and proliferation. Under physiological conditions, the endothelium produce several growth factors such HGF, FGF-2 and ECMs (laminins and collagens), which can induce β-cells proliferation, and enhances insulin secretion. In response to the stimuli to maintain an adequate vascular supply islets release, VEGF-A, angiopoietin-1 and insulin, which serves as signal and strongly interact with endothelial cells to proliferate and regulate the blood flow. The native islets received a high vascular supply and undergone to prolong ischemia when are subjected to islet isolation. Thus, isolated islet function may be seriously comprised until revascularization is complete. Islet revascularization is critical step, which can lead to islet survival and successful graft function or graft failure. An ideal islet engraftment must provide with adequate oxygen and nutrient supplies, and supporting extracellular matrix in order to maintain long-term results. To assess both safety and function of the graft accessibility and less invasive methods should be explored. Its application may provide with valuable insights toward the therapeutic benefit of islet transplantation.
ABBREVIATIONS
- ECM
Extracellular Matrices
- eNOS
endothelial Nitric Oxide Synthase
- FGF-2
Fibroblast Growth Factor-2
- HGF
Hepatocyte Growth Factor
- IEQ
islet equivalent
- NO
Nitric Oxide
- SMV
Superior Mesenteric Vein
- VEGF-A
Vascular Endothelial Growth Factor-A
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
ACKNOWLEDGMENTS
We thank to our colleagues in the CA-533 University of Guadalajara for the valuable suggestions and comments during the weekly academic sessions.
Funding
This work was supported in part by Grant-in-Aid for Scientific Research to Jorge David Rivas-Carrillo, M.D. Ph. D. through Consejo Nacional de Ciencia y Tecnologia (CONACyT) 2016-231902.
REFERENCES
- 1.Dolensek J, Rupnik MS, Stozer A. Structural similarities and differences between the human and the mouse pancreas. Islets 2015; 7:e1024405; PMID:26030186; http://dx.doi.org/ 10.1080/19382014.2015.1024405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go VLW. The pancreas: biology, pathobiology, and disease. New York: Raven Press; 1993 [Google Scholar]
- 3.Stendahl JC, Kaufman DB, Stupp SI. Extracellular matrix in pancreatic islets: relevance to scaffold design and transplantation. Cell Transplant 2009; 18:1-12; PMID:19476204; http://dx.doi.org/ 10.3727/096368909788237195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pepper AR, Gala-Lopez B, Ziff O, Shapiro AM. Revascularization of transplanted pancreatic islets and role of the transplantation site. Clin Dev Immunol 2013; 2013:352315; PMID:24106517; http://dx.doi.org/ 10.1155/2013/352315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cano DA, Hebrok M, Zenker M. Pancreatic development and disease. Gastroenterology 2007; 132:745-762; PMID:17258745; http://dx.doi.org/ 10.1053/j.gastro.2006.12.054 [DOI] [PubMed] [Google Scholar]
- 6.Jabs N, Franklin I, Brenner MB, Gromada J, Ferrara N, Wollheim CB, Lammert E. Reduced insulin secretion and content in VEGF-a deficient mouse pancreatic islets. Exp Clin Endocrinol Diabetes 2008; 116(Suppl 1):S46-49; PMID:18777454; http://dx.doi.org/ 10.1055/s-2008-1081486 [DOI] [PubMed] [Google Scholar]
- 7.Alvarez-Perez JC, Ernst S, Demirci C, Casinelli GP, Mellado-Gil JM, Rausell-Palamos F, Vasavada RC, Garcia-Ocana A. Hepatocyte growth factor/c-Met signaling is required for beta-cell regeneration. Diabetes 2014; 63:216-223; PMID:24089510; http://dx.doi.org/ 10.2337/db13-0333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demirci C, Ernst S, Alvarez-Perez JC, Rosa T, Valle S, Shridhar V, Casinelli GP, Alonso LC, Vasavada RC, Garcia-Ocana A. Loss of HGF/c-Met signaling in pancreatic beta-cells leads to incomplete maternal beta-cell adaptation and gestational diabetes mellitus. Diabetes 2012; 61:1143-1152; PMID:22427375; http://dx.doi.org/ 10.2337/db11-1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivas-Carrillo JD, Navarro-Alvarez N, Soto-Gutierrez A, Okitsu T, Chen Y, Tabata Y, Misawa H, Noguchi H, Matsumoto S, Tanaka N, et al. . Amelioration of diabetes in mice after single-donor islet transplantation using the controlled release of gelatinized FGF-2. Cell Transplant 2006; 15:939-944; PMID:17299999; http://dx.doi.org/ 10.3727/000000006783981323 [DOI] [PubMed] [Google Scholar]
- 10.Rivas-Carrillo JD, Soto-Gutierrez A, Navarro-Alvarez N, Noguchi H, Okitsu T, Chen Y, Yuasa T, Tanaka K, Narushima M, Miki A, et al. . Cell-permeable pentapeptide V5 inhibits apoptosis and enhances insulin secretion, allowing experimental single-donor islet transplantation in mice. Diabetes 2007; 56:1259-1267; PMID:17287463; http://dx.doi.org/ 10.2337/db06-1679 [DOI] [PubMed] [Google Scholar]
- 11.Brennan DC, Kopetskie HA, Sayre PH, Alejandro R, Cagliero E, Shapiro AM, Goldstein JS, DesMarais MR, Booher S, Bianchine PJ. Long-Term Follow-Up of the Edmonton Protocol of Islet Transplantation in the United States. Am J Transplant 2016; 16:509-517; PMID:26433206; http://dx.doi.org/ 10.1111/ajt.13458 [DOI] [PubMed] [Google Scholar]
- 12.Pepper AR, Gala-Lopez B, Pawlick R, Merani S, Kin T, Shapiro AM. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat Biotechnol 2015; 33:518-523; PMID:25893782; http://dx.doi.org/ 10.1038/nbt.3211 [DOI] [PubMed] [Google Scholar]
- 13.Brissova M, Fowler M, Wiebe P, Shostak A, Shiota M, Radhika A, Lin PC, Gannon M, Powers AC. Intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets. Diabetes 2004; 53:1318-1325; PMID:15111502; http://dx.doi.org/ 10.2337/diabetes.53.5.1318 [DOI] [PubMed] [Google Scholar]
- 14.Oh BJ, Oh SH, Jin SM, Suh S, Bae JC, Park CG, Lee MS, Lee MK, Kim JH, Kim KW. Co-transplantation of bone marrow-derived endothelial progenitor cells improves revascularization and organization in islet grafts. Am J Transplant 2013; 13:1429-1440; PMID:23601171; http://dx.doi.org/ 10.1111/ajt.12222 [DOI] [PubMed] [Google Scholar]
- 15.Quaranta P, Antonini S, Spiga S, Mazzanti B, Curcio M, Mulas G, Diana M, Marzola P, Mosca F, Longoni B. Co-transplantation of endothelial progenitor cells and pancreatic islets to induce long-lasting normoglycemia in streptozotocin-treated diabetic rats. PLoS One 2014; 9:e94783; PMID:24733186; http://dx.doi.org/ 10.1371/journal.pone.0094783 [DOI] [PMC free article] [PubMed] [Google Scholar]


