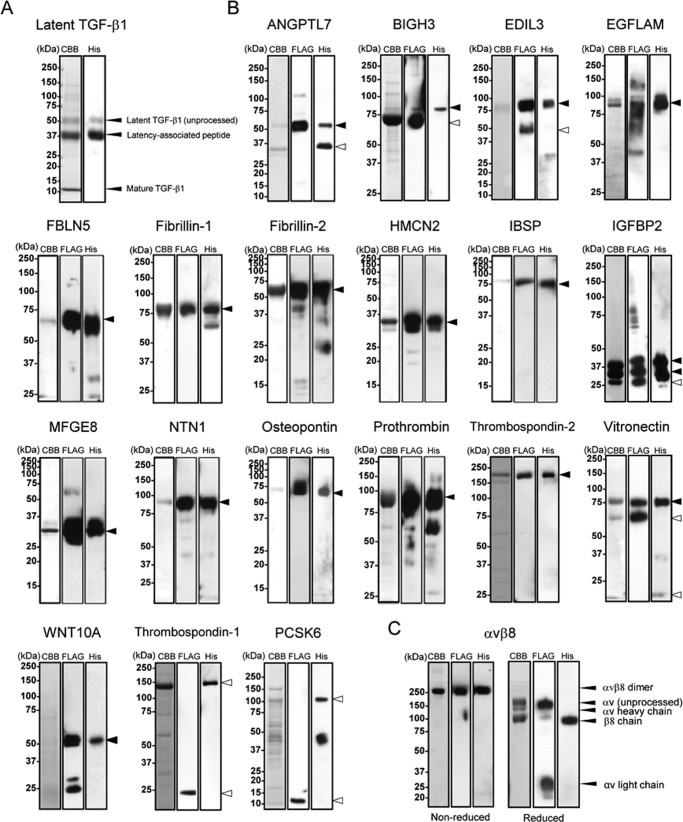
FIGURE 1.
Purification of recombinant RGD proteins and αvβ8 integrin. Purified latent TGF-β1 (A), other recombinant RGD proteins (B), and αvβ8 integrin (C) were subjected to SDS-PAGE on 5–20% gradient gels (ANGPTL7, EDIL3, PCSK6, and latent TGF-β1), 8% gels (EGFLAM, BIGH3, FBLN5, IGFBP2, NTN1, prothrombin, thrombospondin-2, vitronectin, WNT10A, thrombospondin-1, and αvβ8 integrin), or 12% gels (fibrillin-1, fibrillin-2, HMCN2, IBSP, MFGE8, and osteopontin) under reducing conditions, followed by Coomassie Brilliant Blue staining (left), immunoblotting with an anti-FLAG monoclonal antibody (middle), or with an anti-penta-His monoclonal antibody (right), except for αvβ8 integrin that was analyzed under both reducing and non-reducing conditions. Molecular masses are indicated on the left of panels. Arrowheads indicate predicted molecular size of full-length (close) or processed form (open) of each recombinant protein.