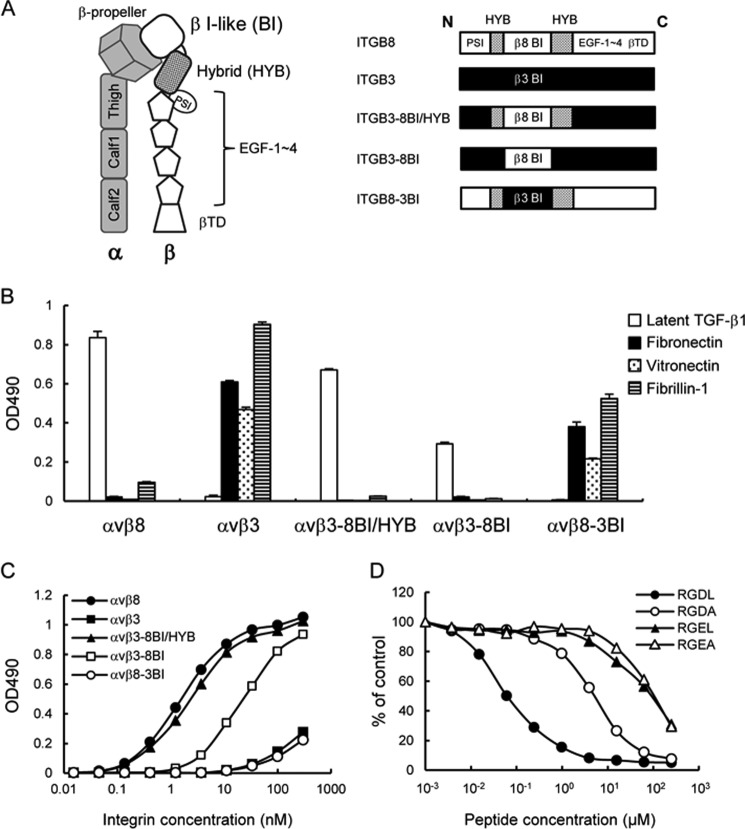
FIGURE 6.
Ligand-binding specificities of domain swap mutants of αvβ8 and αvβ3 integrins. A, schematic of the ectodomain of integrin (left) and representations of the β8/β3 swap mutants (right). The β8- and β3-derived domains are represented by open boxes and closed boxes, respectively. The β8 hybrid domain is represented by dotted boxes. B, binding activities of domain swap mutants of αvβ8 and αvβ3 integrins toward latent TGF-β1, fibronectin, vitronectin, and fibrillin-1. C, titration curves of swap mutants bound to latent TGF-β1. Increasing concentrations of αvβ8 integrin (closed circles), αvβ3 integrin (closed squares), αvβ3–8BI/HYB (closed triangles), αvβ3–8BI (open squares), and αvβ8–3BI (open circles) were allowed to bind to microtiter plates coated with latent TGF-β1 in the presence of 1 mm MnCl2. Bound integrins were quantified as described under “Experimental Procedures.” The results represent the means of three independent determinations. Apparent dissociation constants of recombinant integrins are summarized in Table 3. D, inhibition of αvβ3–8BI binding to latent TGF-β1 by synthetic peptides. The assays were performed as described in the Fig. 4 legend. The results represent the means of three independent determinations. Closed circles, RGDL (9-mer containing both RGD motif and Leu residue); open circles, RGDA (9-mer with the Leu → Ala substitution); closed triangles, RGEL (9-mer with RGD → RGE substitution); open triangles, RGEA (9-mer with RGDL → RGEA double substitution).