FIGURE 8.
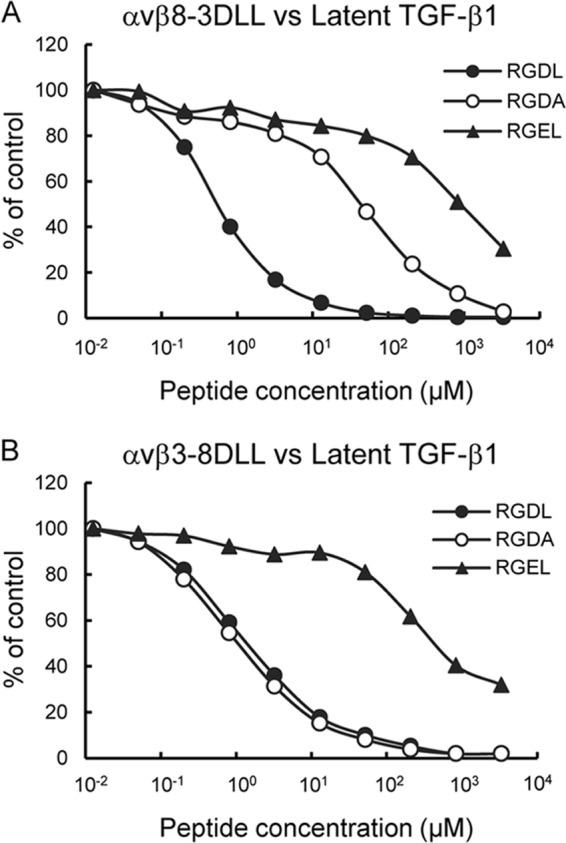
Inhibition of DLL swap mutant binding to latent TGF-β1 by synthetic peptides. αvβ8–3DLL (A) and αvβ3–8DLL (B) mutants (10 nm) were incubated on microtiter plates coated with latent TGF-β1 (10 nm) in the presence of increasing concentrations of synthetic peptides. To prevent precipitation of the peptides, the integrin binding assays were performed in the presence of 10% DMSO. The amounts of bound integrins are shown as percentages relative to the control, in which integrins were incubated on latent TGF-β1-coated plates in the presence of 10% DMSO. The results represent the means of three independent determinations. Closed circles, RGDL (9-mer containing both RGD motif and Leu residue); open circles, RGDA (9-mer with the Leu → Ala substitution); closed triangles, RGEL (9-mer with RGD → RGE substitution).
