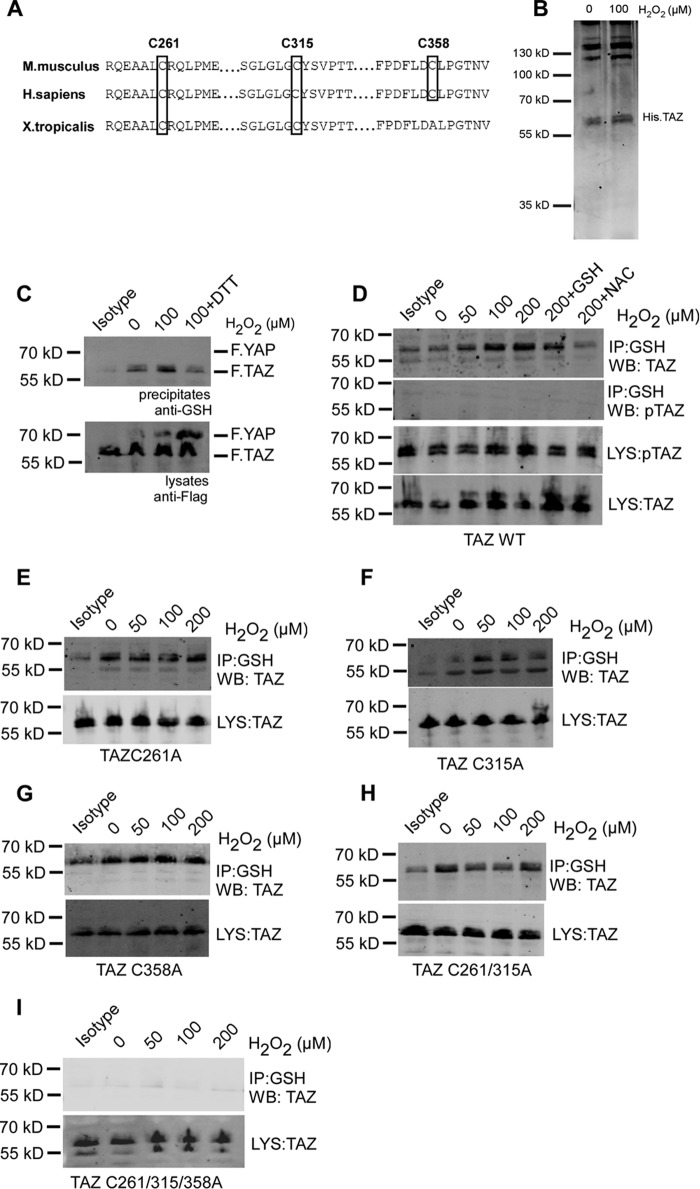
FIGURE 2.
H2O2 induces S-glutathionylation of TAZ at three cysteine residues. A, sequence alignment of mouse TAZ (NP_598545.2) reveals conserved cysteine residues at position Cys-261, Cys-315, and Cys-358. B, Coomassie staining of recombinant His.TAZ protein following 100 μm H2O2 exposure for 30 min in vitro. C, HEK 293T cells transiently co-transfected with FLAG.TAZ and FLAG.YAP were treated with 100 μm H2O2 for 1 h. 1 mm DTT was added to cells 30 min after H2O2 exposure. After immunoprecipitation (IP) with anti-GSH antibody, Western blotting analysis revealed that wild-type FLAG.TAZ (55 kDa) undergoes S-glutathionylation but not YAP (72 kDa). D, HEK 293T cells transiently transfected with FLAG.TAZ were treated with 50, 100, and 200 μm H2O2 for 1 h. After IP with anti-GSH antibody, Western blotting (WB) analyses of lysates (LYS) and precipitates (IP) revealed that wild-type FLAG.TAZ undergoes S-glutathionylation in a dose-dependent manner, which was inhibited by the antioxidants GSH and N-acetylcysteine. E–I, HEK 293T cells transiently transfected with FLAG.TAZ cysteine mutants as indicated were treated with 50, 100, and 200 μm H2O2 for 1 h. After IP with anti-GSH antibody, Western blotting analysis revealed that TAZ undergoes S-glutathionylation in all mutants analyzed (C261A (E), C315A (F), C358A (G), C261A/C315A (H), and C261A/C315A/C358A (I)), indicating the presence of multiple S-glutathionylation sites within TAZ. Mouse IgG (isotype) is shown as a control. Shown are representative data from three independent experiments.