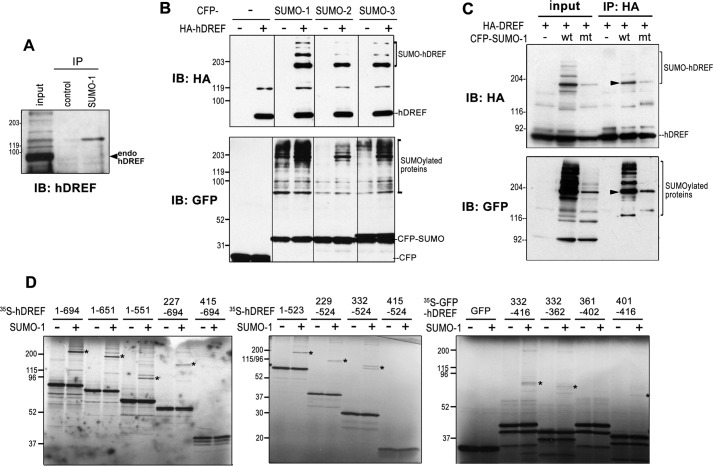
FIGURE 1.
hDREF is SUMOylated. A, endogenous hDREF is conjugated with SUMO-1. Proteins in HeLa cell extract were immunoprecipitated (IP) with anti-SUMO-1 polyclonal antibody and subjected to immunoblotting analysis (IB) with anti-hDREF antibody. The data are representative of two independent experiments with similar results. B, HeLa cells were cotransfected with HA-hDREF and CFP-SUMO expression plasmids as indicated. HA-hDREF polypeptides with or without SUMO conjugation and SUMOylated endogenous proteins were detected by immunoblotting analysis with anti-HA and anti-GFP antibodies, respectively. The data are representative of two independent experiments with similar results. C, HeLa cells were cotransfected with HA-hDREF plasmid and CFP-SUMO-1 plasmids (wild-type (wt) or G97A mutant (mt) SUMO-1) as indicated. HA-hDREF polypeptides were precipitated with anti-HA antibody and analyzed by immunoblotting using an anti-HA antibody (top). SUMOylated proteins coimmunoprecipitated with HA-hDREF were also detected using anti-GFP antibody (bottom). The data are representative of two independent experiments with similar results. Arrowheads, represent HA-hDREF with CFP-SUMO-1 conjugation. D, in vitro SUMOylation assay using a set of hDREF deletion mutants labeled with [35S]methionine. hDREF was synthesized by cell-free coupled in vitro transcription/translation in the presence of [35S]methionine and subjected to an in vitro SUMOylation reaction containing 2 μg of GST-SUMO-1(GG), 1 μg of GST-Ubc9, 0.5 μg of GST-Uba2, 0.5 μg of GST-Aos1 in 50 mm Tris-HCl (pH 8.0), 5 mm MgCl2, 5 mm ATP, and 1 mm dithiothreitol at 37 °C for 30 min. Reactions were terminated by heating the samples in Laemmli's sample buffer at 85 °C for 3 min. The samples were resolved by SDS-PAGE, and signals were detected by autoradiography. *, SUMOylated 35S-hDREF. The data are representative of two independent experiments with similar results.