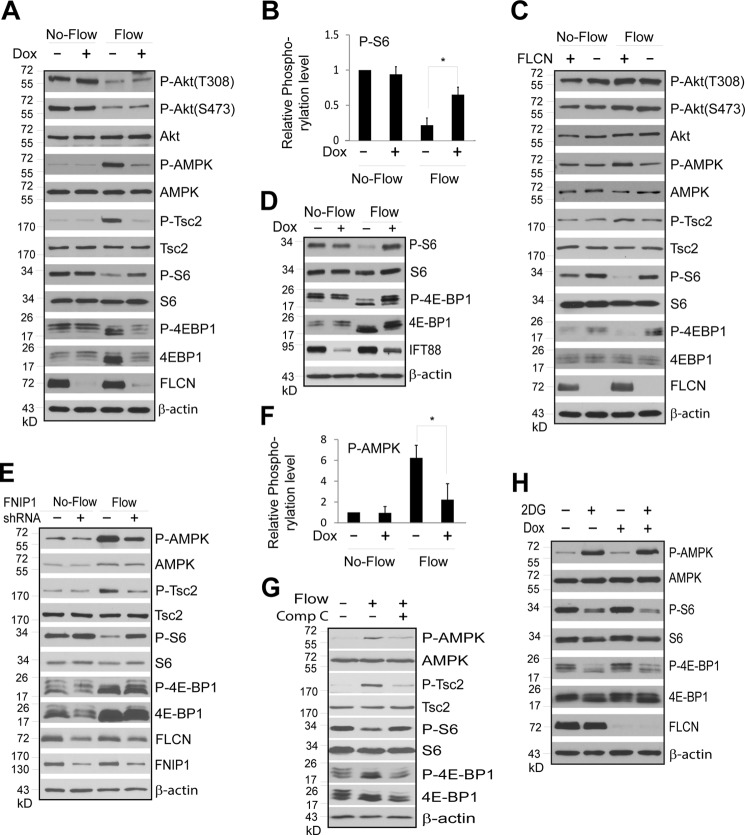
FIGURE 3.
FLCN regulates mTORC1 and AMPK activity in response to flow stress. FLCN-deficient and their wild type control cells were subjected to no-flow or flow stress. Total and phosphorylation levels of indicated proteins in the cells were examined by Western blotting. A, ciliated HKC-8 cells stably expressing doxycycline inducible FLCN shRNA were grown in the absence (Dox −) or presence (Dox +) of doxycycline under flow or no-flow condition. B, quantitative presentation of the relative phosphorylation levels of S6 shown in A (*, p < 0.01). The relative phosphorylation level was expressed as the ratio between the phosphorylation and protein levels that was normalized against that of the control cells grown in the absence of doxycycline and flow stress. Data were from four independent experiments. C, ciliated UOK257–2 (FLCN +) and UOK257 (FLCN −) cells were grown under flow or no-flow condition. D, ciliated HKC-8 cells stably expressing doxycycline inducible IFT88 shRNA were grown in the presence (Dox +) or absence (Dox −) under flow or no-flow condition. E, ciliated HKC-8 cells stably expressing scrambled control (FNIP1 shRNA−) or FNIP1 specific (FNIP1 shRNA+) were subjected to flow or no-flow condition. F, quantitative presentation of the relative phosphorylation levels of AMPK shown in A (*, p < 0.01). The relative phosphorylation level was expressed as the ratio between the phosphorylation and protein levels that was normalized against that of the control cells grown in the absence of doxycycline and flow stress. Data were from four independent experiments. G, HKC-8 cells were grown under flow (Flow +) or no-flow (Flow −) condition for 5 days and treated with (Comp C +) or without (Comp C −) AMPK inhibitor Compound C at a concentration of 10 μm (EMD Millipore) for 24 h. H, HKC-8 cells stably expressing doxycycline inducible FLCN shRNA were grown in the presence (Dox +) or absence (Dox −) for 3 days followed by treatment with 20 mm of 2-deoxyglucose (2DG +) or vehicle control (2DG−) for 2 h.