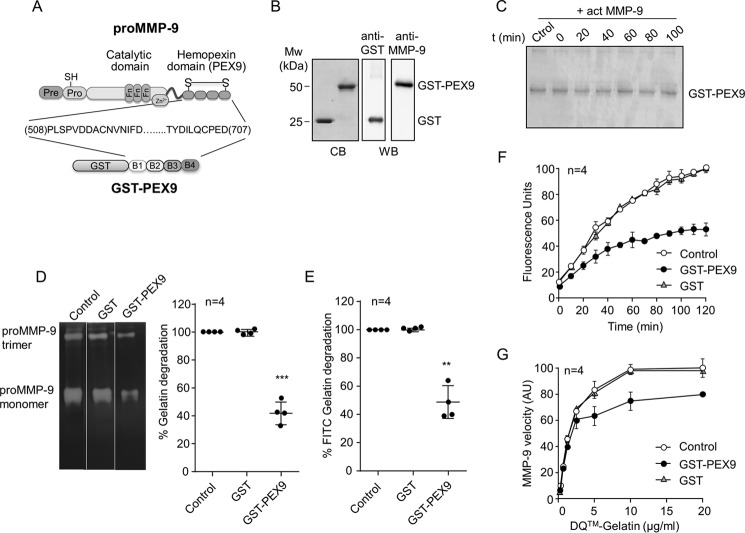
FIGURE 1.
Effect of human GST-PEX9 on the degradation of gelatin by MMP-9. A, schematic drawing of the structural domains of proMMP-9. The four structural blades of PEX9 (B1–B4) are indicated. The GST-PEX9 fusion protein prepared in this study contains amino acid residues 508–707 of human proMMP-9. B, SDS-PAGE (10% acrylamide) and Western blotting (WB) analyses of the purified GST and GST-PEX9 proteins. C, 1 μg of GST-PEX9 was incubated with 1 nm active MMP-9 for the indicated times. The samples were analyzed by 10% SDS-PAGE and stained with Coomassie Blue (CB). D, gelatin zymography analysis of 20 ng of MMP-9 (within the linearity of the assay) in the presence or absence of 0.4 μm GST-PEX9. A representative zymography is shown. E, 60 nm of MMP-9 was added to FITC-gelatin-coated plates, in the absence or presence of 0.4 μm GST or GST-PEX9. After 24 h at 37 °C, fluorescence was determined, and control (Ctrol) values were normalized to 100. F, effect of GST and GST-PEX9 (0.4 μm) on the conversion of DQ-gelatin into fluorogenic gelatin. G, maximal enzyme velocity as a function of the amount of substrate. The maximal velocity of each point is the first read time point at 10 min, and these measurements were within the linearity of the assay. The bars represent standard deviation. AU, arbitrary units. **, p < 0.01; ***, p < 0.001.