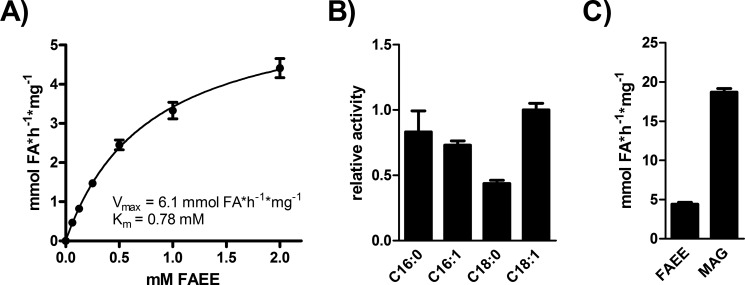
FIGURE 3.
FAEE hydrolase activity of purified Yju3p. A, substrate saturation of purified Yju3p. Yju3p was incubated with substrates containing different concentrations of ethyl palmitate for 30 min. B, FAEE hydrolase activity of purified Yju3p against different FAEE species. Yju3p was incubated with 2 mm FAEE containing different acyl chains. The release of FA was determined by a coupled colorimetric assay. Data are presented as relative activities compared with C18:1 FAEE. C, comparison of FAEE and MAG hydrolysis catalyzed by purified Yju3p. Yju3p was incubated with 2 mm ethyl palmitate or palmitoylglycerol as substrate. Vmax and Km were determined by nonlinear regression analysis. Assays were performed in triplicate and are representative of two independent experiments. Data are presented as the means ± S.D.