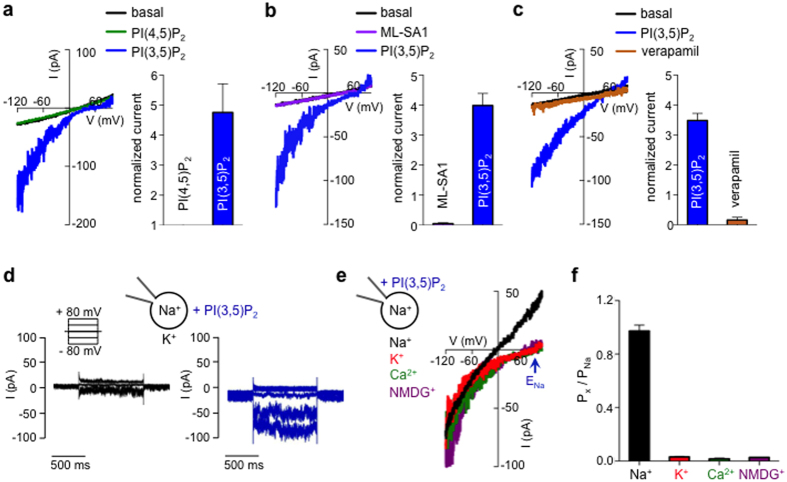
Figure 2. PI(3,5)P2 activates a TPC-like current.
(a) In a representative melanosome from an Oa1−/− melanocyte, bath application of the intracellular organelle-specific PI(3,5)P2 (2 μM), but not the same concentration of plasma membrane PI(4,5)P2, activated IPIP2. Average PIP2-induced increase in current density was measured at −120 mV and normalized to basal currents recorded before PI(4,5)P2 or PI(3,5)P2 treatment (± s.e.m., n = 3 melanosomes, p < 0.01 for PI(4,5)P2- vs. PI(3,5)P2-elicited currents). Gluc−-based luminal solutions were used to block outwardly rectifying Cl−-dependent currents while measuring inward IPIP2. (b) 2 μM PI(3,5)P2 but not the TRPML1 agonist ML-SA1 (25 μM) activated IPIP2 in a representative melanosome. Average fold increase in current density measured at −120 mV (± s.e.m., n = 3 melanosomes, p < 0.001 for PI(3,5)P2- vs. ML-SA1-elicited currents). (c) In a representative melanosome, IPIP2 was inhibited by the TPC antagonist verapamil (150 μM). Average fold increase in current density measured at −120 mV (± s.e.m., n = 4 melanosomes, p < 0.0001 for IPIP2 vs. IPIP2 in the presence of verapamil). (d) In a representative melanosome, PI(3,5)P2 activated an inward current in response to negative voltage steps; no significant PI(3,5)P2-dependent currents were elicited by depolarizing voltages (representative of 3 melanosomes, p < 0.001 for basal currents vs IPIP2). (e) PI(3,5)P2 activated-currents were selective for Na+, but not K+, Ca2+, or the impermeant cation NMDG+. Currents were recorded from the same melanosome with 140 mM luminal NaGluc while substituting symmetrical concentrations of cytoplasmic K+, NMDG+, or 100 mM Ca2+ in a Gluc-based solution. (f) IPIP2 permeability ratios based on Erev measurements (± s.e.m., n = 4 melanosomes, p < 0.0001 for K+, NMDG+ or Ca2+ vs. Na+).