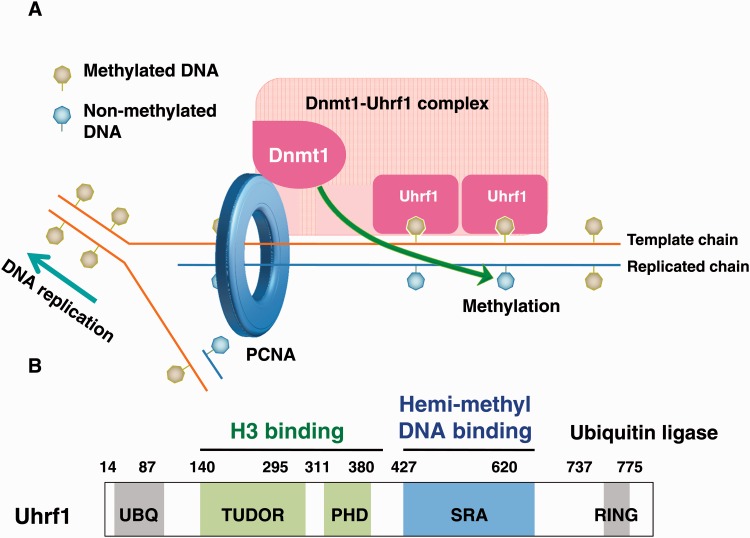
Fig. 2.
Uhrf1 plays an essential role in the recruitment of Dnmt1 to hemi-methylated DNA regions during DNA replication. (A) Methylated cytosine cannot be replicated by DNA replication machinery, generating hemi-methylated DNA. Uhrf1 could specifically bind to hemi-methylated DNA. Uhrf1 then recruits Dnmt1 around the sites of hemi-methylated DNA although molecular mechanism underlying this recruitment remains to be elusive. The recruited Dnmt1 then catalyzes the conversion of himi-metylated DNA to full methylated DNA. (B) Uhrf1 contains various unique domains. TUDOR and PHD domains function as a single unit for readout of H3K9 methylation. SRA domain is a hemi-methylated DNA binding domain, flipping 5-methylcytosine out of the DNA helix. The carboxyl-terminal RING finger domain ubiquitylates H3K23 in a DNA replication-dependent manner. The amino-terminal ubiquitin like domain (UBQ) presents a highest homology with ubiquitin molecule, but its function remains elusive.