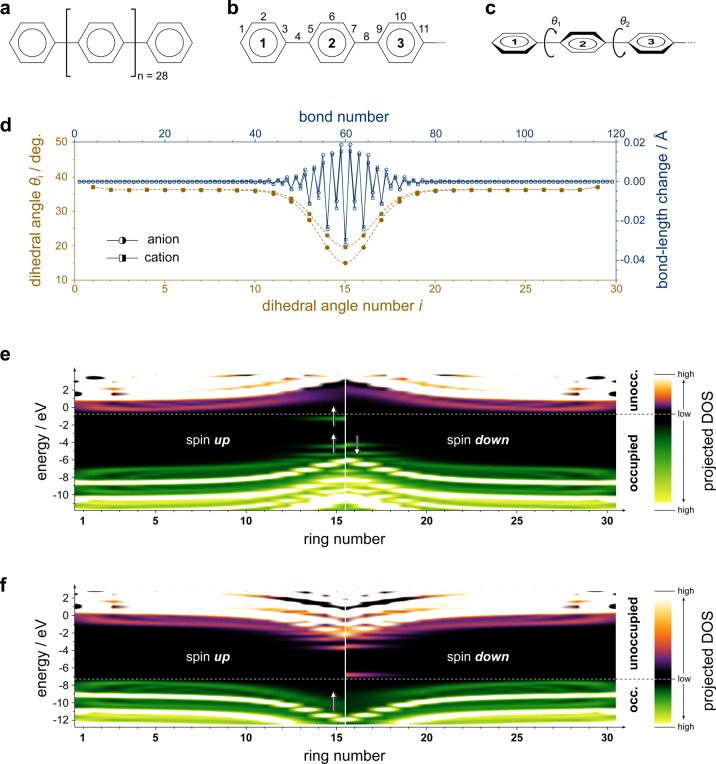
Figure 2.
Charge-induced structural relaxation and electronic density of states in a prototypical polymer. (a) Chemical structure of the investigated compound. (b) Schematic illustrating the numbering of bonds. (c) Schematic illustrating the numbering and sign of the interring dihedral angles; bold numbers refer to the numbering of rings. (d) Dihedral angles θi (in degrees) in the anion (brown, filled circles) and in the cation (brown, filled squares); connecting lines are spline interpolations to guide the eye. Also shown are the changes in bond lengths upon adding (blue, open circles) and removing (blue, open squares) an electron from the neutral oligomer. (e) Calculated density of states (DOS) for the radical anion, projected onto individual rings. The spin-up channel is shown for rings 1 through 15 and the spin-down channel for rings 16 through 30. The respective other half of the oligomer is symmetry equivalent. The color scheme indicates the magnitude of the projected DOS from low (black) to high (white) as well as its occupation (green and violet tones for occupied and unoccupied, respectively). The horizontal, white, dashed line marks the boundary between the occupied and the unoccupied states. The white arrows indicate electrons with according spin to facilitate juxtaposition to Figure 1. (f) Same as (e) for the radical cation.