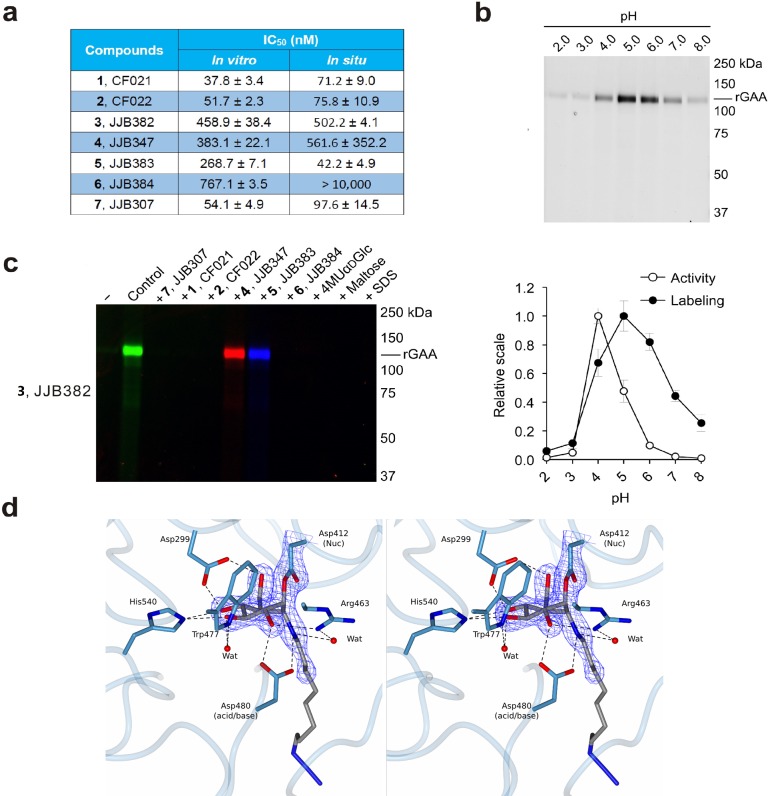
Figure 3.
In vitro inhibition and labeling of α-glucosidases. (a) Inhibition of recombinant GAA. Apparent IC50 values (extrapolated with one phase exponential association) are the mean of three separate experiments. Error ranges depict standard deviation. (b) Labeling of rGAA with compound 3 at various pH values as compared to activity toward 4-MU-α-d-glucopyranose. Error bars represent standard deviation. (c) ABP labeling of rGAA with 3 competed with compounds 1, 2, and 4–7. (d) Stereoscopic view of the CjAgd31B active site in complex with compound 2, showing covalent link to CjAgd31B enzymatic nucleophile Asp412, and H-bonding interactions to neighboring residues. Electron density is REFMAC maximum-likelihood/σA-weighted 2 Fo – Fc synthesis contoured at 0.49 electrons per Å3.